Oral vs Intravenous Administration: A Comparative Pharmacokinetic Guide for Drug Development
This article provides a comprehensive analysis of the pharmacokinetic principles governing oral and intravenous drug administration, tailored for researchers and drug development professionals.
Oral vs Intravenous Administration: A Comparative Pharmacokinetic Guide for Drug Development
Abstract
This article provides a comprehensive analysis of the pharmacokinetic principles governing oral and intravenous drug administration, tailored for researchers and drug development professionals. It explores the foundational mechanisms of drug absorption and bioavailability, examines advanced methodological tools like PBPK modeling, addresses key challenges in oral formulation, and presents validation strategies for comparative drug performance. By integrating current research and case studies, this review serves as a strategic resource for optimizing drug delivery systems and informing clinical development decisions.
Core Principles: Understanding Fundamental Pharmacokinetic Differences Between Oral and IV Routes
Absolute bioavailability is a fundamental pharmacokinetic parameter that quantifies the fraction of an orally administered drug that reaches the systemic circulation unchanged relative to intravenous administration. This critical metric reflects the combined impact of absorption, intestinal metabolism, and hepatic first-pass effects on drug disposition. Intravenous administration serves as the gold standard benchmark for these calculations because it delivers the complete drug dose directly into systemic circulation, bypassing absorption barriers and presystemic metabolism. This review synthesizes experimental data and methodological approaches from clinical studies to compare oral versus intravenous administration, providing researchers with a framework for evaluating drug delivery efficiency and optimizing therapeutic formulations.
Absolute bioavailability (F) represents the systemic availability of a drug administered via a non-intravenous route compared to intravenous administration. The calculation of absolute bioavailability relies on comparing the area under the concentration-time curve (AUC) after non-IV administration to the AUC after IV dosing, with appropriate dose normalization [1]. This parameter is crucial in drug development because it reflects the composite effects of incomplete absorption and presystemic metabolism, both of which limit a drug's therapeutic potential.
Intravenous administration provides the ideal reference point for bioavailability calculations because it achieves 100% systemic availability by delivering the drug directly into the circulation [2]. When a drug is administered orally, it must overcome numerous barriers before reaching systemic circulation, including dissolution in the gastrointestinal fluid, permeation through the intestinal mucosa, and metabolism in the gut wall and liver before entering the systemic bloodstream. The extent of this "first-pass effect" varies considerably among drugs and represents a key determinant of oral dosage formulation [2].
Understanding absolute bioavailability enables researchers to make critical decisions about drug developability, dosing regimens, and formulation strategies. Drugs with low absolute bioavailability often exhibit high inter-individual variability, require higher doses to achieve therapeutic effects, and present greater risk of adverse events due to nonlinear pharmacokinetics. Consequently, accurately determining this parameter through well-controlled clinical trials remains an essential component of pharmacokinetic characterization during drug development.
Quantitative Comparison of Absolute Bioavailability Across Drug Classes
The absolute bioavailability of orally administered drugs varies considerably across different therapeutic classes and molecular entities. This variability stems from differences in chemical structure, solubility, permeability, and susceptibility to metabolic enzymes and transport proteins. The following table summarizes absolute bioavailability values for several drugs determined in controlled clinical studies comparing oral and intravenous administration.
Table 1: Absolute Bioavailability Values for Selected Orally Administered Drugs
| Drug | Therapeutic Category | Absolute Bioavailability | Key Factors Influencing Bioavailability | Study Reference |
|---|---|---|---|---|
| BI 425809 | Glycine transporter-1 inhibitor | 71.64% | Moderate first-pass metabolism | [1] |
| Ibuprofen | NSAID | 91% | High permeability, minimal first-pass effect | [3] |
| Maraviroc | CCR5 antagonist | 23.1% | Extensive metabolism, P-glycoprotein efflux | [2] |
| FK 506 (Tacrolimus) | Immunosuppressant | 25% (mean) | Variable absorption, bile-dependent | [4] |
| Tramadol | Opioid analgesic | 80% | Moderate first-pass metabolism | [3] |
The data reveal a spectrum of bioavailability values, with ibuprofen demonstrating high bioavailability (91%) due to its favorable permeability and minimal first-pass metabolism [3]. In contrast, maraviroc shows substantially lower bioavailability (23.1%) attributable to its extensive metabolism and involvement of efflux transporters [2]. BI 425809 falls in the intermediate range with approximately 72% bioavailability, suggesting moderate presystemic elimination [1].
These differences in bioavailability have direct clinical implications. Drugs with high bioavailability typically exhibit more predictable dose-response relationships, while those with low or variable bioavailability often require more complex dosing regimens and therapeutic drug monitoring. Furthermore, understanding these differences helps guide formulation strategies, with prodrug approaches often employed for compounds with particularly poor bioavailability.
Experimental Protocols for Determining Absolute Bioavailability
Clinical Study Designs
Determining absolute bioavailability requires carefully controlled clinical trials that compare systemic exposure after oral and intravenous administration. The most common approach involves crossover studies where each participant receives both the oral and intravenous formulations in separate periods with adequate washout intervals. These studies typically employ open-label, single-period, single-arm designs in healthy volunteers under fasting conditions to standardize gastrointestinal variables [1].
For example, in the study of BI 425809, researchers used an innovative IV microtracer approach. Participants received a single oral dose of 25 mg unlabeled BI 425809, followed four hours later (coinciding with expected tmax) by an intravenous microtracer infusion containing 30 μg of [14C]-BI 425809 [1]. This design allowed simultaneous characterization of both routes while minimizing radioactive exposure. The use of radiolabeled IV doses enabled researchers to differentiate between the two administrations through accelerator mass spectrometry, providing precise concentration measurements despite the vastly different doses [1].
Pharmacokinetic Sampling and Analysis
Comprehensive blood sampling protocols are essential for accurate AUC determination. In bioavailability studies, sampling typically begins before dose administration and continues through the complete elimination phase. For instance, in the BI 425809 study, plasma samples for oral drug analysis were collected at 2 hours pre-dose and at 1, 2, 3, 4:05, 6, 8, 12, 24, 72, 120, and 168 hours after administration [1]. For the IV microtracer, additional early time points were included (4:10, 4:15, 4:30 hours) to characterize the distribution phase accurately [1].
The primary pharmacokinetic parameters calculated in these studies include AUC from zero to infinity (AUC0-∞), AUC from zero to the last quantifiable concentration (AUC0-t), and maximum plasma concentration (Cmax). Absolute bioavailability (F) is then calculated using the formula:
F (%) = (AUCoral × DoseIV) / (AUCIV × Doseoral) × 100%
This calculation normalizes for dose differences between administration routes. Statistical comparisons typically use geometric mean ratios with 90% confidence intervals, with bioequivalence generally accepted when these intervals fall within 80-125% [5].
Visualization of Absolute Bioavailability Determination
The following diagram illustrates the conceptual framework and experimental workflow for determining absolute bioavailability in clinical studies.
Determining Absolute Bioavailability: Framework and Workflow
This diagram illustrates the parallel pathways of oral and intravenous drug administration in bioavailability studies. The oral route involves absorption and potential first-pass metabolism before reaching systemic circulation, while intravenous administration provides direct systemic access. The experimental components—study design, pharmacokinetic sampling, and analytical methods—support the generation of AUC values needed for the final bioavailability calculation.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 2: Key Research Reagents and Materials for Bioavailability Studies
| Reagent/Material | Function in Bioavailability Studies | Example from Literature |
|---|---|---|
| Radiolabeled drug compounds ([14C]-labeled) | Enable precise tracking of drug disposition; facilitate IV microtracer approaches | [14C]-BI 425809 used as IV microtracer [1] |
| Validated bioanalytical assays (LC-ESI-MS/MS) | Quantify drug concentrations in biological matrices with high sensitivity and specificity | LC-ESI-MS/MS method for Eperisone quantification with LLOQ of 0.01 ng/mL [6] |
| Accelerator Mass Spectrometry (AMS) | Detect extremely low levels of radiolabeled compounds; essential for microtracer studies | AMS used to measure [14C]-BI 425809 concentrations following IV microtracer dose [1] |
| Enzyme-linked Immunosorbent Assay (ELISA) Kits | Quantify biologic drugs and protein therapeutics in biological samples | ELISA kit used to measure serum hCG concentrations in bioequivalence study [5] |
| Standardized formulation components | Ensure consistent drug delivery for both oral and intravenous routes | Hydroxy propyl methyl cellulose used in FK 506 capsule formulation [4] |
| 1-Octanol | 1-Octanol, CAS:220713-26-8, MF:C8H18O, MW:130.23 g/mol | Chemical Reagent |
| Oxalacetic acid | Oxalacetic Acid Reagent|For Research Use | High-purity Oxalacetic Acid (OAA) for life science research. A key metabolic cycle intermediate for biochemistry, cell biology, and disease studies. For Research Use Only. Not for human consumption. |
The selection of appropriate research reagents and analytical methodologies is critical for generating reliable bioavailability data. Advanced analytical techniques such as LC-ESI-MS/MS provide the sensitivity and specificity needed to characterize drug concentration-time profiles accurately, especially for drugs administered at low doses or those with extensive distribution [6]. Similarly, the use of radiolabeled compounds coupled with AMS detection enables researchers to conduct innovative study designs like the IV microtracer approach, which provides comprehensive pharmacokinetic information while minimizing participant exposure to radioactivity [1].
The determination of absolute bioavailability using intravenous administration as a benchmark remains an essential component of pharmacokinetic characterization in drug development. The experimental approaches and comparative data presented in this review demonstrate the critical importance of this parameter for understanding drug disposition and optimizing therapeutic regimens. The varying bioavailability values observed across different drug classes highlight the profound impact of physiological processes on drug delivery and underscore the need for rigorous, well-controlled clinical studies to accurately characterize this fundamental pharmacokinetic property. As drug development advances toward more challenging molecular targets, the principles and methodologies of absolute bioavailability determination will continue to provide crucial insights for translating preclinical candidates into effective human therapeutics.
Oral administration is the most common and preferred route for drug delivery due to its non-invasiveness, patient compliance, and cost-effectiveness [7]. However, the journey of an orally administered drug from the gastrointestinal (GI) tract to the systemic circulation is complex, facing numerous physicochemical and biological barriers. Understanding the mechanisms governing this process—passive diffusion, carrier-mediated transport, and efflux pumps—is fundamental to pharmaceutical research and development, particularly in comparative pharmacokinetics where oral bioavailability is benchmarked against the complete systemic availability of intravenous administration [8] [9].
For a drug to be effective after oral administration, it must dissolve in GI fluids, survive the harsh acidic and enzymatic environment, cross the intestinal epithelial membrane, and withstand first-pass metabolism in the liver before reaching systemic circulation [9] [7]. This article provides a comparative guide to the core mechanisms of oral drug absorption, supporting researchers with structured data and experimental protocols to advance drug delivery science.
Core Mechanisms of Oral Drug Absorption
Passive Diffusion: The Dominant Pathway
Definition and Principle: Passive diffusion is the most common mechanism for drug absorption, driven by the concentration gradient across the intestinal membrane according to Fick's law of diffusion [8] [10]. In this process, drug molecules move from a region of high concentration (the GI lumen) to a region of low concentration (the bloodstream) without energy expenditure [11].
Key Determinants:
- Lipid Solubility: The lipoid nature of cell membranes favors the diffusion of lipophilic drugs [11].
- Degree of Ionization: The fraction of un-ionized drug is critical for permeability, governed by the drug's pKa and the environmental pH via the Henderson-Hasselbalch equation [8] [10] [11]. For instance, weakly acidic drugs like aspirin exist primarily in un-ionized, absorbable forms in the stomach, while weak bases are better absorbed in the higher pH of the small intestine [11].
- Molecular Size: Smaller molecules diffuse more rapidly than larger ones [11].
Table 1: Key Characteristics of Passive Diffusion
| Parameter | Description | Research Implication |
|---|---|---|
| Driving Force | Concentration gradient | Independent of energy; follows Fick's Law [10] |
| Membrane Route | Transcellular | Requires optimal lipophilicity (Log P) [8] |
| pH Dependency | High (for weak electrolytes) | Requires pH-pKa profiling for predictive modeling [11] |
| Saturability | Non-saturable | Linear kinetics typically observed [8] |
Carrier-Mediated Transport: Facilitated and Active
Definition and Principle: Certain drugs that are structurally similar to endogenous nutrients (e.g., amino acids, sugars, vitamins) utilize specialized carrier proteins embedded in the intestinal membrane [8] [11]. This system is characterized by specificity, saturability, and competitive inhibition.
Types of Carrier Transport:
- Active Transport: Requires energy (ATP) to move drugs against their concentration gradient. This process is selective for drugs mimicking natural metabolites, such as the antihyperglycemic agent metformin, which is transported by the organic cation transporter 1 (OCT1) [8] [12].
- Facilitated Diffusion: Utilizes carrier proteins to move drugs along their concentration gradient without energy expenditure. An example is the transport of vitamin B12 [11].
Table 2: Comparison of Carrier-Mediated Transport Mechanisms
| Characteristic | Active Transport | Facilitated Diffusion |
|---|---|---|
| Energy Requirement | Required (ATP-dependent) [8] | Not required [11] |
| Direction of Transport | Against concentration gradient [8] | Along concentration gradient [11] |
| Saturability | Yes [8] | Yes [8] |
| Example Drug/Transporter | α-Methyldopa [12]; Cephalexin/PEPT1 [12] | Vitamin B12 transport [11] |
Efflux Pumps: P-glycoprotein and Absorption Limitation
Definition and Principle: Efflux transporters act as biological barriers by actively pumping absorbed drugs back into the intestinal lumen, thereby limiting their systemic bioavailability [8] [10]. The most extensively studied efflux pump is P-glycoprotein (P-gp), an ATP-binding cassette (ABC) transporter [8] [10].
Functional Impact:
- P-gp is expressed on the apical surface of intestinal epithelial cells and functions as an ATP-dependent efflux pump for a wide range of structurally diverse drugs [8] [10].
- It significantly restricts the absorption of many drugs, including anticancer agents (e.g., paclitaxel), HIV protease inhibitors, and cardiovascular drugs [10]. Research in rats shows that co-administration with a P-gp inhibitor (HM30181) increased paclitaxel's oral bioavailability from 3.4% to 41.3% [10].
The following diagram illustrates the dynamic interplay of these three primary mechanisms in the intestinal epithelial cell.
Experimental Models for Investigating Absorption Mechanisms
Choosing an appropriate experimental model is critical for accurately studying oral drug absorption. The following table compares the primary models used in research, highlighting their advantages and limitations [13].
Table 3: Comparison of Experimental Models for Studying Oral Absorption
| Model Type | Advantages | Limitations | Ideal Application |
|---|---|---|---|
| In Vivo (Whole Animal) | Most accurately simulates human physiology; evaluates long-term effects and overall bioavailability [13]. | Long cycle, high cost, ethical concerns, individual variability, difficulty isolating specific pathways [13]. | Preclinical efficacy verification; studying systemic effects on absorption [13]. |
| Ex Vivo/In Situ Tissue | Retains key intestinal structures and functions (barriers, transporters); shorter cycle and lower cost than in vivo [13]. | Lacks systemic factors (blood flow); limited tissue viability; requires specialized operation [13]. | Analyzing nanoparticle-epithelium interactions; screening formulation effects on intestinal barriers [13]. |
| In Vitro (Cell Models) | Low cost, highly controllable, enables molecular-level studies; no animal ethics issues [13]. | Lacks key physiology (mucus, microbiota); simplified cell structure; may overestimate permeability [13]. | Early high-throughput screening; studying endocytic pathways and intracellular transport [13]. |
The Caco-2 Cell Model: The human colon adenocarcinoma cell line (Caco-2) is the most widely used in vitro model. When cultured as a monolayer, these cells spontaneously differentiate to exhibit many characteristics of small intestinal epithelium, including microvilli, tight junctions, and functional expression of various transporters (e.g., P-gp) and metabolic enzymes [13] [12]. Before experimentation, the integrity of the monolayer must be validated, typically by measuring transepithelial electrical resistance (TEER) or using marker molecules [13].
Research Reagents and Methodologies
The Scientist's Toolkit: Essential Reagents
Table 4: Key Research Reagent Solutions for Absorption Studies
| Reagent / Solution | Function in Research | Example Application |
|---|---|---|
| Caco-2 Cells | A human intestinal epithelial cell line that forms polarized monolayers with microvilli, transporters, and tight junctions, mimicking the intestinal barrier [13] [12]. | Standard model for predicting passive transcellular and carrier-mediated drug permeability [13]. |
| Transport Inhibitors | Chemical compounds used to block specific transport pathways to study their contribution (e.g., P-gp inhibitors like verapamil or GF120918) [13]. | Elucidating the role of efflux pumps; studying the mechanism of carrier-mediated uptake [13]. |
| Simulated Intestinal Fluids (FaSSIF/FeSSIF) | Biorelevant media mimicking the fasted (FaSSIF) and fed (FeSSIF) state intestinal fluid, containing bile salts and phospholipids [14]. | Studying dissolution, supersaturation, and the effect of bile micelles on drug solubility and permeability [14]. |
| Endocytosis Inhibitors | Inhibitors of specific endocytic pathways (e.g., chlorpromazine for clathrin-mediated endocytosis) used to study nanoparticle uptake [13]. | Investigating the cellular internalization mechanisms of nano-formulations [13]. |
| μFLUX System | An in vitro dissolution-permeation flux system that simultaneously monitors drug dissolution and permeation in real-time [14]. | Predicting food effects and the interplay between solubility and permeability [14]. |
| Sulcatone | Sulcatone, CAS:409-02-9, MF:C8H14O, MW:126.20 g/mol | Chemical Reagent |
| Ucf-101 | Ucf-101, CAS:5568-25-2, MF:C27H17N3O5S, MW:495.5 g/mol | Chemical Reagent |
Detailed Experimental Protocol: μFLUX for Food Effect Prediction
The μFLUX system is a key tool for evaluating how food impacts the absorption of drugs, particularly those whose absorption is limited by both solubility and epithelial membrane permeation (SL-E drugs) [14].
Methodology:
- Preparation of Donor Solutions: Prepare fasted-state simulated intestinal fluid (FaSSIF) and fed-state simulated intestinal fluid (FeSSIF) according to established biorelevant recipes. These solutions differ primarily in their bile salt and phospholipid content, mimicking in vivo conditions [14].
- Drug Addition: The model drug (e.g., Bosentan, Pranlukast) is introduced to the donor compartment in its solid form or as a suspension.
- Permeation Membrane: A permeation barrier, typically a Caco-2 cell monolayer or an artificial membrane, separates the donor and acceptor compartments.
- Flux Measurement: The system is maintained at 37°C with continuous agitation. Samples are taken from the acceptor compartment over time and analyzed via HPLC or LC-MS/MS to determine the drug flux (JμFLUX), which represents the rate of drug permeation [14].
Data Interpretation:
- For SL-E drugs, even though FeSSIF may drastically increase total drug solubility (Sdissolv) due to bile micelle solubilization, the critical parameter is the free drug concentration (Sdissolv,u), which often remains unchanged. Consequently, the observed JμFLUX may show little enhancement despite a large increase in total dissolved drug, correctly predicting a weak food effect [14].
- This experimental observation validates the theoretical framework that for SL-E drugs, an increase in solubility by bile micelles is counterbalanced by a decrease in effective permeability [14].
The workflow for this integrated experimental and theoretical approach is summarized below.
The absorption of oral drugs is a multifaceted process governed primarily by passive diffusion, carrier-mediated transport, and efflux pumps. A deep understanding of these mechanisms is indispensable for rational drug design and formulation strategies aimed at overcoming poor bioavailability. The interplay of these processes determines the fraction of the administered dose that ultimately reaches the systemic circulation, a key parameter in comparative pharmacokinetics against intravenous administration.
Advanced experimental models and biorelevant tools, such as the Caco-2 cell system and the μFLUX apparatus, provide critical insights into these mechanisms. By integrating theoretical frameworks like FaRLS with robust experimental data, researchers can more accurately predict in vivo performance, including the impact of food, leading to the development of more effective and reliable oral drug products.
First-pass metabolism, also known as the first-pass effect, is a fundamental pharmacokinetic phenomenon wherein a drug undergoes pre-systemic metabolism at specific locations in the body before reaching the systemic circulation or its site of action [15]. This process substantially decreases the concentration of the active drug, thereby limiting its therapeutic potential when administered via the oral route. While the liver serves as the primary site for first-pass metabolism, other metabolically active tissues including the gastrointestinal lumen, gastrointestinal wall, and lungs also contribute to this effect [15] [16].
The clinical impact of first-pass metabolism is profound, as it represents one of the most significant barriers to oral drug bioavailability. For drug development scientists and researchers, understanding the mechanisms and extent of first-pass elimination is crucial for optimizing drug delivery systems and dosing regimens. The variability of first-pass metabolism among individuals—influenced by factors such as age, gender, genetic polymorphisms, and disease states—further complicates the prediction of in vivo drug performance from in vitro models [15] [17]. This article examines the experimental evidence quantifying first-pass effects and compares the pharmacokinetic profiles of drugs administered via oral versus intravenous routes, providing researchers with methodological frameworks for studying this critical pharmacological barrier.
Physiological Mechanisms and Metabolic Pathways
The first-pass effect encompasses several sequential barriers that orally administered drugs must navigate before entering the systemic circulation. The process begins in the gut lumen, where enzymes and the acidic environment can degrade certain pharmaceutical compounds [16]. Subsequently, drugs face metabolic challenges within the enterocytes of the intestinal wall, which contain significant concentrations of cytochrome P450 enzymes, particularly CYP3A4, and phase II conjugation enzymes [16] [18].
After surviving intestinal metabolism, drugs are transported via the portal vein to the liver, where they encounter highly efficient enzyme systems capable of extensive biotransformation [15] [17]. The hepatic metabolism involves Phase I reactions (oxidation, reduction, hydrolysis) primarily mediated by cytochrome P450 enzymes, and Phase II reactions (conjugation with glucuronide, sulfate, or other moieties) that typically render compounds more water-soluble for excretion [17]. The interplay between these metabolic pathways determines the extent to which a drug survives first-pass metabolism to produce its therapeutic effect.
The following diagram illustrates the sequential metabolic barriers that constitute the first-pass effect for orally administered drugs:
Quantitative Comparison: Oral vs. Intravenous Administration
Direct pharmacokinetic comparisons between oral and intravenous administration provide the most compelling evidence of first-pass metabolism. The table below summarizes key experimental findings from clinical studies that quantify these differences across multiple therapeutic agents:
Table 1: Pharmacokinetic Comparison of Oral versus Intravenous Drug Administration
| Drug | Study Details | Key Pharmacokinetic Findings | Bioavailability Impact |
|---|---|---|---|
| Chloramphenicol [19] | 14 infants with H. influenzae meningitis; 100 mg/kg/day every 6 hours | - IV: Mean peak serum level: 15.0 μg/ml at 45 min- Oral: Mean peak serum level: 18.5 μg/ml at 2-3 hours- Serum half-life: 4.0 hours (IV) vs. 6.5 hours (oral) | Extended half-life but delayed Tmax with oral administration |
| Busulfan [20] | 40 patients undergoing hematopoietic stem cell transplantation | - IV: Median AUC: 1244.8 μMol·min- Oral: Median AUC: 1174 μMol·min- Extreme interpatient variability in oral pharmacokinetics | Similar AUC but higher variability with oral administration |
| Diazepam [15] | Pediatric patients with febrile seizures | Active metabolites (desmethyldiazepam, oxazepam) with similar physiological effects | Significant first-pass metabolism with active metabolites |
| Nitroglycerin [15] | Angina treatment | Rapid relief via sublingual administration bypassing first-pass effect | Complete avoidance of first-pass metabolism with alternative routes |
| Dextromethorphan [15] | Pseudobulbar affect treatment | Coadministration with quinidine inhibits first-pass metabolism | 45-50% increase in systemic exposure with metabolic inhibition |
The data demonstrate that first-pass metabolism not only reduces drug bioavailability but can also significantly alter other pharmacokinetic parameters, including time to peak concentration (Tmax) and elimination half-life. These changes have profound implications for dosing regimen design and therapeutic monitoring protocols.
Experimental Models for Studying First-Pass Metabolism
Research into first-pass metabolism employs a hierarchy of experimental models, each with distinct advantages and limitations. The following table systematizes the primary models used in absorption and first-pass metabolism research:
Table 2: Experimental Models for First-Pass Metabolism Research
| Model Type | Advantages | Limitations | Applications |
|---|---|---|---|
| In Vivo (Whole Animal) [13] | - Most accurately simulates human physiology- Evaluates long-term effects and systemic bioavailability- Suitable for preclinical verification | - Long experimental cycles and high costs- Individual variability reduces reproducibility- Ethical considerations- Difficulty isolating specific pathways | - Studying systemic absorption effects- Long-term bioavailability studies- Preclinical efficacy verification |
| Ex Vivo/In Situ Intestinal Models [13] | - Retains key intestinal structures and functions- Less complex than whole animal models- Shorter experimental cycles- Direct observation of formulation behavior | - Lacks systemic factors (blood flow, hormones)- Limited tissue viability (hours to days)- Requires specialized isolation techniques- Microenvironment alterations may distort results | - Analyzing local intestinal absorption mechanisms- Screening formulation effects on barriers- Comparing segmental absorption differences |
| Cell Models (Caco-2, MDCK) [13] | - Low cost, easy standardization, high controllability- Enables molecular-level mechanism studies- No animal ethical issues- High-throughput screening capability | - Lacks key physiological components (mucus, microbiota)- Simplified cell structure not fully representative- Cannot simulate multi-organ interactions- May overestimate permeability | - Early formulation screening- Molecular-level mechanism studies- Transporter-mediated uptake investigations |
The workflow for employing these models in first-pass metabolism studies typically follows a sequential approach from simple screening to complex validation:
Methodological Approaches for Investigating First-Pass Effects
Clinical Pharmacokinetic Study Protocol
The standard methodology for quantifying first-pass metabolism in human subjects involves a crossover design comparing oral and intravenous administration [19]:
Subject Selection: Recruit appropriate patient populations or healthy volunteers with consideration for genetic profiling of metabolic enzymes
Dosing Protocol:
- Intravenous administration with precise dosing and infusion rate documentation
- Oral administration after an appropriate washout period, typically with standardization of fasting/fed state
Sample Collection:
- Serial blood sampling at predetermined time points (e.g., pre-dose, 0.25, 0.5, 1, 2, 4, 6, 8, 12, 24 hours post-dose)
- Plasma separation via centrifugation and storage at -80°C until analysis
Bioanalytical Methods:
- Utilization of validated LC-MS/MS methods for drug and metabolite quantification
- Implementation of stable isotope-labeled internal standards for precision
Data Analysis:
- Non-compartmental analysis to determine AUC, Cmax, Tmax, and t1/2
- Calculation of absolute bioavailability: F = (AUCoral × DoseIV) / (AUCIV × Doseoral)
- Assessment of metabolite-to-parent drug ratios to evaluate metabolic pathways
Advanced Research Techniques
Emerging technologies are addressing the limitations of traditional models in studying first-pass metabolism [13]:
- Microphysiological Systems: Organ-on-a-chip platforms that simulate human gastrointestinal and hepatic tissues with fluidic connectivity
- CRISPR-Cas9 Modified Cell Lines: Genetically engineered intestinal and hepatic cells with specific overexpression or knockout of metabolic enzymes and transporters
- In Vivo Subcellular High-Resolution Imaging: Advanced microscopy techniques to track drug distribution and metabolism in real-time at the subcellular level
- Physiologically Based Pharmacokinetic (PBPK) Modeling: Computational approaches that integrate in vitro metabolism data with physiological parameters to predict first-pass extraction
Research Reagent Solutions for First-Pass Metabolism Studies
Table 3: Essential Research Reagents for First-Pass Metabolism Investigations
| Reagent/Category | Specific Examples | Research Applications |
|---|---|---|
| Cell Models [13] | Caco-2 cells, MDCK cells, Primary hepatocytes, HuTu-80, HepG2 | Intestinal permeability screening, hepatic metabolism studies, transporter characterization |
| Enzyme Inhibitors [15] [17] | Ketoconazole (CYP3A4), Quinidine (CYP2D6), Sulfaphenazole (CYP2C9), BNPP (esterases) | Metabolic pathway identification, enzyme contribution quantification, drug-interaction studies |
| Transport Inhibitors [18] | Verapamil (P-gp), MK-571 (MRP2), Ko143 (BCRP) | Transporter efflux assessment, absorption enhancement strategies |
| Specialized Polymers [21] | HPMC, HPMCAS, PVP, PVP-VA, PEG | Amorphous solid dispersions, solubility enhancement, crystallization inhibition |
| Permeation Enhancers [18] | Sodium caprate, Chitosan, Medium-chain fatty acids, Labrasol | Paracellular pathway modulation, tight junction regulation, absorption improvement |
| Analytical Standards [19] [20] | Deuterated internal standards, metabolite standards, certified reference materials | Bioanalytical method development, metabolite identification and quantification |
Strategies to Overcome First-Pass Metabolic Barriers
Multiple formulation and chemical strategies have been successfully employed to mitigate the impact of first-pass metabolism:
Formulation Approaches
Prodrug Design: Chemical modification of drug molecules to create precursors that bypass first-pass metabolism but convert to active forms in systemic circulation [16]. Examples include ester prodrugs that are resistant to hepatic hydrolysis but undergo plasma enzyme conversion.
Lymphatic Targeting: Utilization of lipid-based formulations including self-emulsifying drug delivery systems (SEDDS) that promote intestinal lymphatic transport, thereby bypassing hepatic first-pass metabolism [18].
Nanoparticle Systems: Engineered nanocarriers that protect drugs from metabolic degradation and enhance absorption through specialized pathways, including M-cell transport in Peyer's patches [13] [21].
Permeation Enhancers: Excipients that temporarily increase intestinal permeability by modulating tight junctions or fluidizing epithelial cell membranes [18].
Alternative Administration Routes
Sublingual and Buccal Delivery: Administration through the oral mucosa directly into the systemic circulation, completely avoiding first-pass metabolism [15] [16].
Rectal Administration: Partial avoidance of first-pass metabolism through hemorrhoidal vein drainage directly into the inferior vena cava [15].
Transdermal Systems: Continuous delivery through the skin into systemic circulation, bypassing gastrointestinal and hepatic metabolism [16].
First-pass metabolism remains a critical determinant of oral drug bioavailability with profound implications for drug development and clinical practice. The experimental evidence clearly demonstrates that first-pass effects can substantially reduce systemic drug exposure, alter metabolic profiles, and increase interpatient variability. For researchers engaged in drug development, a multifaceted approach combining advanced in vitro models, preclinical validation, and strategic formulation design offers the most promising path to overcoming these metabolic barriers.
The continuing evolution of research methodologies—including microphysiological systems, targeted genomic editing, and sophisticated computational modeling—promises to enhance our predictive capabilities regarding first-pass metabolism. By systematically applying these tools and strategies, drug development scientists can more effectively navigate the challenges posed by first-pass effects and optimize the therapeutic potential of novel pharmaceutical compounds.
Drug absorption describes the transportation of an unmetabolized drug from its site of administration into the systemic circulation [8]. This process is the most fundamental principle in pharmacokinetics and is the critical first step determining whether an active pharmaceutical ingredient will reach its target site of action. The most common mechanisms for this transport include passive diffusion, where drug molecules move according to a concentration gradient from areas of high concentration to low concentration, and carrier-mediated membrane transport, which includes active transport and facilitated diffusion [8]. The complex journey of a drug from its administered form to systemic circulation is influenced by a confluence of factors that can be broadly categorized as drug-specific (physicochemical and pharmaceutical variables) and patient-specific (physiological variables) [8].
The route of administration profoundly impacts the absorption process and the ultimate bioavailability of a drug, defined as the rate and extent of its absorption [8]. Generally, the order of bioavailability among different routes of administration, ranked from highest to lowest, is parenteral, rectal, oral, and topical [8]. Intravenous (IV) administration achieves 100% bioavailability because the drug is introduced directly into the bloodstream, completely bypassing the absorption process [8] [22]. In contrast, oral administration requires the drug to survive the gastrointestinal (GI) tract environment, cross the intestinal epithelium, and withstand first-pass metabolism in the liver before reaching systemic circulation, which often results in reduced and more variable bioavailability [11] [22]. Understanding the interplay between a drug's physicochemical properties and the biological barriers it encounters is essential for predicting and optimizing its therapeutic performance.
The Biopharmaceutics Classification System (BCS): A Framework for Predicting Absorption
The Biopharmaceutics Classification System (BCS) is a fundamental scientific framework that prognosticates the in vivo absorption performance of orally administered drugs based on two key physicochemical properties: solubility and intestinal permeability [23]. Developed for human drug application, this system categorizes drug substances into one of four classes, which helps identify the rate-limiting step in the absorption process and guides formulation strategies [23] [24].
The Four BCS Classes
BCS Class I: High Solubility, High Permeability These drugs are generally very well-absorbed because they readily dissolve in the GI fluids and easily cross the intestinal membrane. Their absorption is typically rapid and complete, and dissolution rate often dictates the absorption speed [23]. For example, a study analyzing drugs on the World Health Organization's Essential Medicines list found that 84% of the classifiable drugs belonged to BCS Class I [24].
BCS Class II: Low Solubility, High Permeability For these compounds, absorption is dissolution rate-limited. Although the drug has high permeability once in solution, its poor solubility slows the dissolution process, thereby restricting the overall rate and sometimes the extent of absorption. Formulation strategies for BCS Class II drugs often focus on enhancing solubility and dissolution, such as through particle size reduction or the use of solubilizing agents [23].
BCS Class III: High Solubility, Low Permeability The absorption of BCS Class III drugs is permeability rate-limited. While these drugs dissolve readily in the GI tract, their ability to cross the intestinal epithelium is low. Consequently, their absorption is highly dependent on the permeability barrier rather than formulation factors affecting dissolution [23].
BCS Class IV: Low Solubility, Low Permeability Drugs in this class suffer from both poor solubility and poor permeability, leading to significant challenges in achieving adequate and consistent oral bioavailability. They often exhibit very poor and highly variable absorption [23].
Table 1: Biopharmaceutics Classification System (BCS) Drug Categories
| BCS Class | Solubility | Permeability | Absorption Characteristic | Rate-Limiting Step |
|---|---|---|---|---|
| Class I | High | High | Very well-absorbed | Gastric emptying / Dissolution rate |
| Class II | Low | High | Dissolution-limited absorption | Dissolution rate |
| Class III | High | Low | Permeability-limited absorption | Intestinal permeability |
| Class IV | Low | Low | Poor and variable absorption | Combination of dissolution and permeability |
Defining High Solubility and High Permeability
The BCS provides specific, standardized criteria for classifying a drug as "highly soluble" or "highly permeable". A drug is considered highly soluble when the highest marketed dose strength is soluble in 250 mL of aqueous media over a pH range of 1.0 to 7.5 at 37°C [23]. This volume is derived from typical human gastric fluid volume. A drug is classified as highly permeable when the extent of intestinal absorption in humans is determined to be 90% or more of an administered dose, based on a mass balance study or in comparison to an intravenous reference dose [23].
The following diagram illustrates the logical decision process for classifying a drug according to the BCS framework:
Experimental Protocols for Key Assays
Determining a drug's BCS classification requires robust and standardized experimental methods to accurately measure its solubility and permeability.
Solubility and Dissolution Assessment
The solubility measurement for BCS classification is dose-dependent, not purely intrinsic. The experimental protocol involves:
- Apparatus: Shaking water bath or equivalent system maintained at 37°C ± 0.5°C.
- Buffer Preparation: Prepare aqueous media within the pH range of 1.0 to 7.5 (e.g., 0.1 N HCl or pH 7.5 phosphate buffer). The pH of the solution should be verified after the addition of the drug substance.
- Procedure: An excess of the drug substance is added to a measured volume of the buffer (typically 250 mL is the benchmark volume for human BCS). The mixtures are shaken for a sufficient time to reach equilibrium (often up to 24 hours).
- Analysis: The solutions are then centrifuged or filtered to remove undissolved material, and the concentration of the drug in the supernatant or filtrate is quantified using a validated stability-indicating assay, such as High-Performance Liquid Chromatography (HPLC) with UV detection [23].
- Calculation: The dose number (Do), a key parameter, is calculated as the ratio of the drug dose to the product of its solubility and a standard volume (250 mL for humans). A Do value greater than 1 classifies the drug as poorly soluble [23].
Permeability Assessment
While human absorption data is the gold standard for permeability classification, several in vitro and in vivo models are used during drug development:
- In Vitro Cell-Based Models: The Caco-2 (human colon adenocarcinoma) cell line is a widely accepted model for predicting human intestinal permeability. Cells are cultured on semi-permeable membranes until they differentiate into an intestinal epithelium-like monolayer. The drug is added to the donor compartment (apical side for simulating intestinal lumen), and samples are taken from the receiver compartment (basolateral side) over time to determine the apparent permeability coefficient (Papp). A good correlation has been shown between permeability coefficients in Caco-2 cells and the fraction of drug absorbed in humans [24].
- In Vivo Pharmacokinetic Studies: Absolute bioavailability (F) studies provide the most direct measure of absorption. In these studies, the exposure (AUC) of a drug after oral administration is compared to its exposure after intravenous administration in the same subject. An absolute bioavailability of 90% or higher is considered evidence of high permeability [23]. For instance, a study comparing intravenous and oral chloramphenicol in infants with meningitis provided key pharmacokinetic data, including half-life and bioavailability, that illuminated its absorption characteristics [19].
Table 2: Experimental Models for Assessing BCS Criteria
| Parameter | Primary Method | Key Experimental System | Classification Threshold |
|---|---|---|---|
| Solubility | Equilibrium Solubility | Shake-flask method in pH 1–7.5 buffers at 37°C | Dose number (Do) ≤ 1 in 250 mL |
| Permeability (In Vitro) | Cell Monolayer Transport | Caco-2 cell model | Papp correlates with ≥90% absorption |
| Permeability (In Vivo) | Mass Balance / Bioavailability | Human pharmacokinetic study | Absolute bioavailability (F) ≥ 90% |
Advanced Considerations and the Role of Precipitation
Beyond the foundational solubility and permeability, other complex phenomena can govern oral absorption. For weakly basic drugs, which represent a significant portion of modern pharmaceuticals, precipitation in the gastrointestinal tract is a critical parameter [25] [26]. These drugs often exhibit high solubility in the acidic environment of the stomach but can precipitate upon entering the more neutral pH of the small intestine, where the majority of absorption occurs. This precipitation can significantly reduce the concentration of drug available for absorption, thereby limiting bioavailability.
Recent research has focused on developing predictive in vitro tools to model this phenomenon. For example, one study developed a miniaturized precipitation assay (µPA) to screen drug candidates during discovery. The results indicated a clear relationship between the extent of precipitation observed in vitro and the fraction of drug absorbed in vivo, highlighting precipitation as a key parameter for early oral absorption predictions [25] [26]. This necessitates an integrated experimental approach that considers solubility, permeability, and precipitation kinetics to build a comprehensive absorption profile, especially for BCS Class II bases.
The following workflow summarizes the integrated experimental strategy for predicting oral drug absorption, incorporating precipitation assessment for basic drugs:
The Scientist's Toolkit: Essential Research Reagents and Materials
The experimental characterization of a drug's absorption potential relies on a suite of specialized reagents, cell lines, and laboratory equipment.
Table 3: Key Research Reagents and Materials for Absorption Studies
| Reagent / Material | Function in Experiment | Application Context |
|---|---|---|
| Caco-2 Cell Line | In vitro model of human intestinal epithelium for permeability screening. | Permeability classification, transport mechanism studies. |
| Transwell Plates | Permeable supports for growing cell monolayers for transport studies. | Caco-2 assays; measuring apparent permeability (Papp). |
| Simulated GI Fluids | (e.g., FaSSGF, FaSSIF) Biorelevant media mimicking fasting-state stomach and intestinal composition. | Solubility and dissolution testing under physiologically relevant conditions. |
| HPLC-UV/MS Systems | High-performance liquid chromatography for quantifying drug concentrations in solubility, dissolution, and permeability samples. | Analytical quantification in all key assays. |
| PAMPA Kit | Parallel Artificial Membrane Permeability Assay for high-throughput passive permeability screening. | Early-stage, high-throughput permeability ranking. |
| Diethyltoluamide | N,N-Diethyl-2-methylbenzamide | N,N-Diethyl-2-methylbenzamide for research applications. This active agent is for Research Use Only. Not for diagnostic, therapeutic, or personal use. |
| Resveratrol | Resveratrol, CAS:133294-37-8, MF:C14H12O3, MW:228.24 g/mol | Chemical Reagent |
The framework established by the Biopharmaceutics Classification System, grounded in the fundamental physicochemical properties of solubility and permeability, provides an indispensable tool for rational drug development. It allows scientists to predict absorption challenges, design appropriate formulations, and understand the implications of in vitro data on in vivo performance. While the BCS was developed for human drugs, its principles are universally applicable, though specific criteria (like the volume for solubility assessment) may require adaptation for veterinary species [23]. The continued evolution of this field, with the integration of more complex phenomena like precipitation and the development of advanced in vitro tools, ensures that the BCS will remain a cornerstone for optimizing the oral bioavailability of new therapeutic agents and for making informed decisions in a regulatory context, including the possibility of biowaivers for BCS Class I drugs [24].
For drug development professionals and clinical pharmacologists, the oral route remains the most common, yet notoriously variable, pathway for drug administration. The fundamental thesis of comparative pharmacokinetics (PK) of oral versus intravenous (IV) administration rests on a simple principle: while IV delivery provides direct access to systemic circulation, oral drugs must navigate a complex gastrointestinal (GI) environment where physiological variables profoundly influence their journey into the bloodstream [27] [8]. This journey—dictated by gastric emptying, intestinal pH, and food interactions—determines the critical pharmacokinetic parameters of bioavailability, time to maximum concentration (Tmax), and overall exposure (AUC). Understanding these variables is not merely academic; it directly informs drug formulation, dosing regimen recommendations, and the interpretation of bioequivalence studies [27] [15]. This guide objectively compares the performance of orally administered drugs against the IV benchmark by examining how core physiological factors alter oral PK, supported by experimental data and methodologies.
Core Physiological Variables Governing Oral Drug Absorption
Gastric Emptying and Intestinal Transit
Gastric emptying is often the rate-limiting step for drug absorption [27]. The transfer of stomach contents into the duodenum is regulated by the migrating motor complex (MMC) during fasting and exhibits a different pattern in the postprandial state [27] [28].
- Fasted State: The MMC cycle, which includes propagating contractions, results in a highly variable gastric emptying time [27].
- Fed State: A high-calorie or high-fat meal can significantly delay gastric emptying, prolonging the time a drug remains in the stomach [27]. This delay directly impacts the Tmax of orally administered drugs.
Small Intestinal Transit Time is typically more consistent, averaging 2-6 hours, and is less influenced by food [29] [28]. This transit determines the time available for dissolution and permeation across the intestinal wall.
Advanced techniques for measuring transit times include:
- Wireless Motility Capsule (WMC): An ingestible, indigestible capsule that measures pH, pressure, and temperature to determine regional transit times [29]. Gastric emptying is marked by an abrupt pH rise as the capsule moves from the acidic stomach to the alkaline duodenum.
- Magnet Tracking System (MTS-1): Tracks an ingested magnet via an external sensor matrix, providing high-resolution data on pill position and contraction frequencies [28]. Pyloric passage is identified by the cessation of the stomach's ~3 contractions/minute pattern and the onset of the small intestine's 8-11 contractions/minute frequency.
Gastrointestinal pH
The pH environment varies significantly along the GI tract and is a major determinant of a drug's dissolution and solubility, particularly for ionizable compounds [27] [30] [8].
Table 1: Physiological pH Ranges in the Gastrointestinal Tract
| GI Segment | Fasted State pH | Fed State pH | Impact on Drug Absorption |
|---|---|---|---|
| Stomach | 1.0 - 3.0 [30] | Transiently rises to ~5.0 [27] | Affects solubility of weak acids/bases; stability of acid-labile drugs. |
| Duodenum | ~6.0 [27] | 6.0 - 8.0 [30] | Primary site for dissolution and absorption for many drugs. |
| Small Intestine | 6.0 - 8.0 [27] [30] | 6.0 - 8.0 [30] | Large surface area favors absorption; bile salts enhance solubility. |
For weakly basic drugs, the elevated gastric pH under fed conditions can reduce dissolution, while for weakly acidic drugs, solubility may increase [30]. Furthermore, acid-reducing agents (ARAs) like proton pump inhibitors can perpetuate these pH-mediated drug-drug interactions [30].
The Impact of Food
Food ingestion triggers a cascade of physiological changes: delayed gastric emptying, increased bile secretion, changes in splanchnic blood flow, and fluctuations in luminal pH [27]. These changes can lead to positive, negative, or neutral food effects on drug absorption.
- Positive Food Effects: Increased bioavailability (AUC) can result from:
- Negative Food Effects: Decreased bioavailability can occur due to:
- Binding or complexation of the drug with food components.
- Instability of the drug in the altered GI environment.
- Delayed Tmax, which may be critical for drugs requiring rapid onset, such as analgesics.
The Biopharmaceutics Classification System (BCS) provides a framework for predicting food effects based on a drug's solubility and permeability [27]. BCS Class II drugs (low solubility, high permeability) are particularly susceptible to food effects, as their absorption is solubility-limited.
Comparative Pharmacokinetics: Oral vs. Intravenous Administration
The intravenous route serves as the gold standard for bioavailability, defined as 100%, because the entire dose enters the systemic circulation directly [8]. In contrast, oral bioavailability (F) is a fraction determined by the extent of absorption and first-pass metabolism.
The relationship is defined by: F = (AUCoral / AUCIV) × (DoseIV / Doseoral)
Table 2: Experimental PK Data from an Ibuprofen/Tramadol Study [31]
| Drug / Analyte | Route | Absolute Bioavailability (F) | Key PK Parameter Observations |
|---|---|---|---|
| Ibuprofen | Oral | 91% | Equivalent AUC for oral and IV administration. |
| Tramadol | Oral | 80% | IV administration produced higher Cmax of parent drug. |
| O-desmethyl-Tramadol (M1) | Oral (as metabolite) | N/A | IV administration of parent Tramadol resulted in lower M1 metabolite exposure than oral route. |
This data illustrates that even with high oral bioavailability, the rate of absorption and metabolite profile can differ significantly between routes, influenced by first-pass metabolism.
The First-Pass Effect
Orally administered drugs are absorbed through the GI tract and enter the hepatic portal vein, passing through the liver before reaching the systemic circulation [15] [16]. Pre-systemic metabolism in the gut wall and liver—the first-pass effect—can substantially reduce the bioavailability of the active drug [15]. Drugs like morphine and propranolol undergo significant first-pass metabolism, necessitating much higher oral than IV doses [15]. This effect exhibits marked inter-individual variability due to differences in enzyme expression (e.g., Cytochrome P450, particularly CYP3A4) and liver function [15] [16].
Diagram 1: First-Pass Effect Pathway.
Methodologies for Assessing Physiological Variables
Experimental Protocols for Transit and Motility
Wireless Motility Capsule (WMC) Protocol [29]:
- Patient Preparation: Overnight fast. Discontinuation of prokinetics, laxatives, narcotics, and anticholinergics 3-7 days prior.
- Test Meal & Ingestion: Ingest a standardized nutrient bar (e.g., SmartBar) and 50 mL water, followed by the activated WMC.
- Post-Ingestion: No food or drink for 6 hours. Patient wears a data receiver for 3-5 days, logging meals, sleep, and bowel movements.
- Data Analysis: Software (e.g., MotiliGI) uses pH, pressure, and temperature data to identify landmarks.
- Gastric Emptying Time (GET): Time from ingestion to an abrupt, sustained pH rise (>3 units).
- Small Bowel Transit Time (SBTT): Time from gastric emptying to an abrupt pH drop (>1 unit) marking entry into the cecum.
- Whole Gut Transit Time (WGTT): Time from ingestion to a temperature drop or signal loss, indicating excretion.
Magnet Tracking System (MTS-1) Protocol [28]:
- Ingestion: A small magnetic pill is swallowed.
- Tracking: An external 4x4 matrix of magnetic field sensors, placed over the abdomen, tracks the pill's position and orientation at 10 Hz.
- Data Analysis: Custom software (e.g., MTS_Record) displays real-time data. Pyloric passage is identified by a change in contraction frequency from ~3/min (stomach) to 8-11/min (small intestine). Ileocecal passage is marked by cessation of the small intestinal frequency pattern.
Assessing Food Effects: Clinical FEED Studies
Regulatory-grade food-effect studies follow a standardized design [27]:
- Design: Single-dose, randomized, two-period (fed vs. fasted), crossover study.
- Fasted State: Overnight fast of at least 10 hours before dosing. No food for at least 4 hours post-dose.
- Fed State: Overnight fast followed by a high-fat, high-calorie meal (e.g., ~800-1000 calories) consumed 30 minutes before dosing.
- PK Analysis: Blood sampling over time to determine AUC, Cmax, and Tmax. A statistical comparison (90% confidence intervals) indicates the presence of a significant food effect.
Diagram 2: Food-Effect Study Workflow.
The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Materials and Tools for GI Transit and Absorption Studies
| Research Tool / Reagent | Function & Application in PK Research |
|---|---|
| Wireless Motility Capsule (WMC) | Provides a non-invasive, radiation-free method for assessing regional and whole gut transit times (GET, SBTT, WGTT) via pH, pressure, and temperature sensors [29]. |
| Magnet Tracking System (MTS-1) | Offers high-resolution tracking of an ingested magnet's position and orientation to determine transit times and contractile frequencies [28]. |
| High-Fat/High-Calorie Meal | Standardized meal per FDA guidance used in food-effect (FEED) studies to create a maximally disruptive physiological state for evaluating food's impact on drug absorption [27]. |
| Simulated Intestinal Fluids | Biorelevant media (e.g., FaSSIF/FeSSIF) that mimic the fasted and fed state composition of human intestinal fluid for in vitro dissolution and solubility testing [32]. |
| PBPK Modeling Software | Physiologically-based pharmacokinetic (PBPK) platforms simulate drug PK under various conditions, integrating drug properties and physiological data to predict food effects and absorption [27] [33]. |
| Capsule Endoscopy (PillCam) | Allows for direct visualization of GI transit and mucosal status; often used as a validation tool for other transit measurement techniques like MTS-1 [28]. |
| Isoalantolactone | Isoalantolactone, CAS:107439-69-0, MF:C15H20O2, MW:232.32 g/mol |
| Stictic acid | Stictic acid, CAS:56614-93-8, MF:C19H14O9, MW:386.3 g/mol |
Advanced Tools and Techniques: PBPK Modeling and Study Designs for Route Comparison
Physiologically-Based Pharmacokinetic (PBPK) Modeling for Predicting Oral Absorption
Predicting oral drug absorption is a critical challenge in drug development, complicated by dynamic physiological processes in the gastrointestinal (GI) tract and complex drug-specific properties. Physiologically-based pharmacokinetic (PBPK) modeling has emerged as a powerful mechanistic tool to simulate and predict the oral absorption process, integrating physiological parameters with drug-specific data to forecast pharmacokinetic profiles [34]. Unlike classical compartmental models that simplify absorption as a first-order process, PBPK models mechanistically represent the GI tract as a series of compartments, each with distinct physiological characteristics that influence drug dissolution, permeation, and metabolism [34]. This approach provides a significant advantage over static prediction methods by enabling dynamic simulation of concentration-time profiles and accounting for complex interplays between drug properties and physiological variables [35].
Within the broader context of comparative pharmacokinetics research, PBPK modeling offers unique insights into the fundamental differences between oral and intravenous administration. While intravenous administration delivers drug directly into systemic circulation, oral absorption involves sequential processes including dissolution, GI transit, permeation, and first-pass metabolism that collectively determine bioavailability [34]. PBPK models quantitatively integrate these processes, allowing researchers to deconvolute the specific factors limiting oral bioavailability and systematically compare exposure profiles across different administration routes.
Comparative Analysis of PBPK Modeling Platforms
PBPK modeling platforms provide specialized software environments that combine technical computational infrastructure with physiological content libraries to support model development and simulation [36]. The leading platforms—including Simcyp Simulator, GastroPlus, and PK-Sim—have evolved to incorporate increasingly sophisticated absorption models that account for regional differences in GI physiology, fluid composition, and transit times [35] [34]. These platforms share a common foundation of integrating system-dependent (physiological) parameters with drug-dependent (physicochemical and ADME) parameters, but differ in their specific implementation approaches, mathematical algorithms, and specialized application modules [36].
A key advancement in PBPK platform development has been the incorporation of compartmental absorption models such as the Advanced Dissolution, Absorption and Metabolism (ADAM) model in Simcyp and the Advanced Compartmental Absorption and Transit (ACAT) model in GastroPlus [35] [34]. These models segment the GI tract into multiple compartments representing different anatomical regions (stomach, duodenum, jejunum, ileum, colon), each with distinct physiological characteristics including pH, surface area, fluid volume, and bile salt concentrations that collectively influence drug absorption [34]. This compartmental approach represents a significant evolution from earlier "mixing tank" models that treated the entire GI tract as a single, well-stirred compartment [34].
Performance Comparison and Validation
Recent studies have systematically evaluated the prediction performance of PBPK platforms for oral absorption, with particular focus on parameter optimization to improve accuracy. A 2025 retrospective analysis of bottom-up PBPK model development at AbbVie using Simcyp Simulator demonstrated how optimization of key system parameters significantly improved prediction accuracy for oral absorption [37].
Table 1: Impact of Parameter Optimization on PBPK Prediction Performance in Simcyp
| Parameter Combination | Cmax Predictions Within 3-fold | AUCINF Predictions Within 3-fold | Key Applications |
|---|---|---|---|
| Original GI physiology + default P-gp REF (1.5) + adjusted ISEF | 43% | 43% | Baseline performance |
| New GI physiology + default P-gp REF (1.5) + adjusted ISEF | 76% | Not specified | Improved absorption for low solubility compounds |
| New GI physiology + P-gp REF (0.5) + default ISEF | 86% | 81% | Optimal for P-gp substrates |
| Original GI physiology + default P-gp REF (1.5) + default ISEF | Not specified | 48% | Improved clearance prediction |
The implementation of "New GI physiology" in Simcyp Version 20, developed through extensive meta-analysis of human intestinal parameters, resulted in substantial improvement in Cmax predictions (76% within 3-fold vs. 43% with Original GI physiology) [37]. This enhancement is particularly valuable for low-solubility compounds whose absorption had been systematically underpredicted [37]. Further optimization of P-glycoprotein Relative Expression Factor (REF) to 0.5 additionally improved Cmax predictions for P-gp substrates to 86% within 3-fold, while using default recombinant CYP enzyme intersystem extrapolation factor (ISEF) values provided the most accurate area under the curve (AUCINF) predictions (81% within 3-fold) [37].
Regulatory applications of PBPK modeling further demonstrate the utility and validation of these platforms. The U.S. Food and Drug Administration has utilized PBPK absorption modeling to support bioequivalence assessments, evaluate food effects, justify biowaivers, and assess the impact of sex differences on drug exposure [38]. In one case example, the FDA employed PBPK modeling to evaluate whether observed differences in in vitro alcohol-induced dose dumping between test and reference products would translate to clinically significant differences in systemic exposure, ultimately determining that the increased release did not pose a safety concern [38].
Critical Parameters for Simulating Oral Absorption
Fundamental Absorption Processes
PBPK models of oral absorption mechanistically represent four fundamental processes that collectively determine the rate and extent of drug absorption: solubility, dissolution, precipitation, and permeation [39]. Each process depends on the interplay between drug-specific properties and physiological conditions throughout the GI tract.
Solubility is not represented as a single value in PBPK models but rather as a dynamic parameter that varies along the GI tract due to regional differences in pH and bile salt concentrations [39]. For weak acids and bases, the pH-dependent ionization significantly influences solubility, with weak bases typically showing higher solubility in the acidic stomach and lower solubility in the near-neutral small intestine [39]. Measuring the pH-solubility profile and bile salt micelle partition coefficient (using media such as FaSSIF) provides critical inputs for PBPK software to calculate regional solubility variations [39].
Dissolution rate determines how quickly solid drug particles dissolve in GI fluids and is commonly described using adaptations of the Noyes-Whitney equation [34]. The dissolution rate depends on solid surface area, solubility, concentration gradient, and fluid hydrodynamics [39]. PBPK platforms incorporate semi-mechanistic dissolution models (z-factor, Wang-Flanagan, Johnson, P-PSD) that translate in vitro dissolution data to predictions of in vivo dissolution by accounting for differences in experimental conditions and physiological parameters [39].
Precipitation may occur when dissolved drug concentrations exceed thermodynamic solubility, particularly for weakly basic drugs that transition from acidic stomach to neutral intestine or for formulations designed to generate supersaturation (e.g., amorphous solid dispersions) [39]. The precipitation rate is drug-dependent and can be determined using transfer tests that simulate the transition from gastric to intestinal conditions [39]. In PBPK models, precipitation is typically simulated using a mean precipitation time (MPT) parameter derived from in vitro experiments [39].
Permeation describes the transport of dissolved drug across the intestinal membrane into systemic circulation and depends on effective permeability (Peff), available surface area, and drug concentration at the absorption site [39] [34]. Human Peff can be predicted using quantitative structure-activity relationship models, measured using cell monolayers (Caco-2, MDCK), or estimated from in situ animal models [39]. PBPK platforms incorporate correlations to translate these experimental measurements to human in vivo permeability values [39].
Parameter Sensitivity Analysis
The relative importance of solubility, dissolution, precipitation, and permeation parameters varies depending on the specific drug, formulation, and physiological conditions [39]. Parameter sensitivity analysis (PSA) within PBPK software identifies which inputs most significantly impact simulation outputs, guiding resource allocation for experimental characterization [39]. For compounds with high permeability and low solubility (BCS Class II), dissolution and solubility parameters typically show high sensitivity, while for compounds with low permeability and high solubility (BCS Class III), permeation parameters dominate absorption variability [39].
Experimental Protocols for PBPK Model Development
Bottom-Up Model Development Workflow
The development of a bottom-up PBPK model for predicting oral absorption follows a systematic workflow that integrates in vitro assay data with physiological parameters. A standardized protocol ensures comprehensive characterization of critical drug properties and appropriate implementation in PBPK platforms.
Table 2: Key Experimental Assays for PBPK Absorption Model Inputs
| Parameter Category | Experimental Assays | Data Application in PBPK Models |
|---|---|---|
| Solubility | pH-solubility profile (pH 1-8); Solubility in FaSSIF/FeSSIF; Solid form characterization | Input for regional solubility calculations; Determination of bile partition coefficient |
| Dissolution | USP Apparatus 1/2; µDiss Profiler; Transfer model systems | Development of biopredictive dissolution method; Determination of dissolution rate constants |
| Permeability | Caco-2/MDCK assays; PAMPA; In situ perfusion | Estimation of human effective permeability (Peff); Identification of transporter involvement |
| Metabolism | Recombinant CYP enzymes; Human liver microsomes; Hepatocytes | Calculation of intrinsic clearance; ISEF optimization for IVIVE |
| Transport | Transporter overexpression systems; Relative expression factors | Quantification of transporter-mediated flux; REF optimization |
Step 1: Compound Characterization - Conduct comprehensive in vitro assays to determine fundamental physicochemical properties including pKa, log P, pH-solubility profile, bile salt partition coefficient, solid form stability, and permeability across biological membranes [39]. For compounds susceptible to metabolism, determine intrinsic clearance using recombinant CYP enzymes (with ISEF adjustment) or human hepatocytes [37].
Step 2: Dissolution Method Development - Establish biopredictive dissolution methods using appropriate apparatus (USP 1, 2, or µDiss Profiler) with media that simulate gastric and intestinal conditions [39]. For compounds with potential precipitation risks, implement transfer tests that simulate the transition from gastric to intestinal environment [39].
Step 3: Model Implementation - Input characterized parameters into PBPK platform, selecting appropriate absorption model (e.g., ADAM in Simcyp, ACAT in GastroPlus) and system parameters (e.g., GI physiology, transporter REF values) [37] [39]. For population simulations, incorporate appropriate variability in physiological parameters (organ volumes, blood flows, enzyme abundances) [36].
Step 4: Model Verification - Conduct parameter sensitivity analysis to identify critical inputs with greatest impact on absorption predictions [39]. Verify model performance against available in vivo data (e.g., preclinical pharmacokinetics) and refine parameters as needed [37] [36].
Step 5: Prediction and Validation - Apply verified model to predict human oral absorption and pharmacokinetics. For regulatory applications, establish model credibility through qualification for specific context of use following established frameworks [36].
Regulatory Qualification Framework
The qualification of PBPK models for regulatory submissions follows a structured framework that encompasses both platform qualification and model validation [36]. Platform qualification demonstrates the predictive capability of the PBPK software for a specific context of use, while model validation verifies that a compound-specific model is appropriately developed and suitable for addressing the regulatory question [36]. The qualification process includes assessment of software integrity, mathematical correctness, algorithm accuracy, and predictive performance for relevant compound classes [36]. For absorption models, qualification typically involves demonstrating accurate prediction of food effects, dose proportionality, formulation differences, and drug-drug interactions at the absorption site [38].
Visualization of PBPK Modeling Workflow
Oral Absorption PBPK Modeling Process
The following diagram illustrates the integrated workflow for developing and applying PBPK models to predict oral absorption, highlighting the sequential processes and key parameters.
PBPK Modeling Workflow for Oral Absorption Prediction
GI Tract Compartmental Structure
The compartmental representation of the gastrointestinal tract in modern PBPK absorption models captures regional variations in physiology that critically influence drug absorption.
GI Tract Compartmental Structure in PBPK Absorption Models
Research Tools and Reagents
Successful implementation of PBPK modeling for oral absorption prediction relies on specialized research tools, software platforms, and experimental systems. The following table catalogues essential solutions used in this field.
Table 3: Essential Research Tools for PBPK Absorption Modeling
| Tool Category | Specific Solutions | Application in PBPK Modeling |
|---|---|---|
| PBPK Software Platforms | Simcyp Simulator, GastroPlus, PK-Sim | Integrated PBPK/PD modeling, population simulations, regulatory submission support |
| In Vitro Dissolution Systems | USP Apparatus 1/2, µDiss Profiler, Transfer Model Systems | Generation of biopredictive dissolution data for model input |
| Permeability Assay Systems | Caco-2 cell models, MDCK cells, PAMPA, In situ perfusion | Determination of effective permeability (Peff) for absorption scaling |
| Solubility & Precipitation Assays | pH-solubility profiling, FaSSIF/FeSSIF, transfer tests | Characterization of regional solubility and precipitation risk |
| Metabolism & Transport Systems | Recombinant CYP enzymes, human hepatocytes, transporter assays | IVIVE of metabolic clearance and transporter interactions |
PBPK modeling represents a sophisticated, mechanistic approach to predicting oral drug absorption that integrates physiological understanding with drug-specific properties. The comparative analysis of PBPK platforms reveals continuous evolution in model sophistication, with recent advances in GI physiology parameters and system-specific scaling factors significantly improving prediction accuracy [37]. The optimal performance achieved with combined parameter optimization (86% of Cmax predictions within 3-fold for P-gp substrates) demonstrates the potential for highly reliable bottom-up predictions when appropriate system parameters are implemented [37].
The utility of PBPK absorption modeling extends throughout the drug development continuum, from early candidate selection to regulatory submission support. For comparative pharmacokinetics research examining oral versus intravenous administration, PBPK models provide a mechanistic framework to deconvolute the complex processes that differentiate these routes, particularly first-pass metabolism and absorption limitations that reduce oral bioavailability [34]. As these models continue to evolve with refined physiological parameters and improved IVIVE approaches, their role in guiding formulation strategy, clinical trial design, and regulatory decisions is expected to expand further [38] [36].
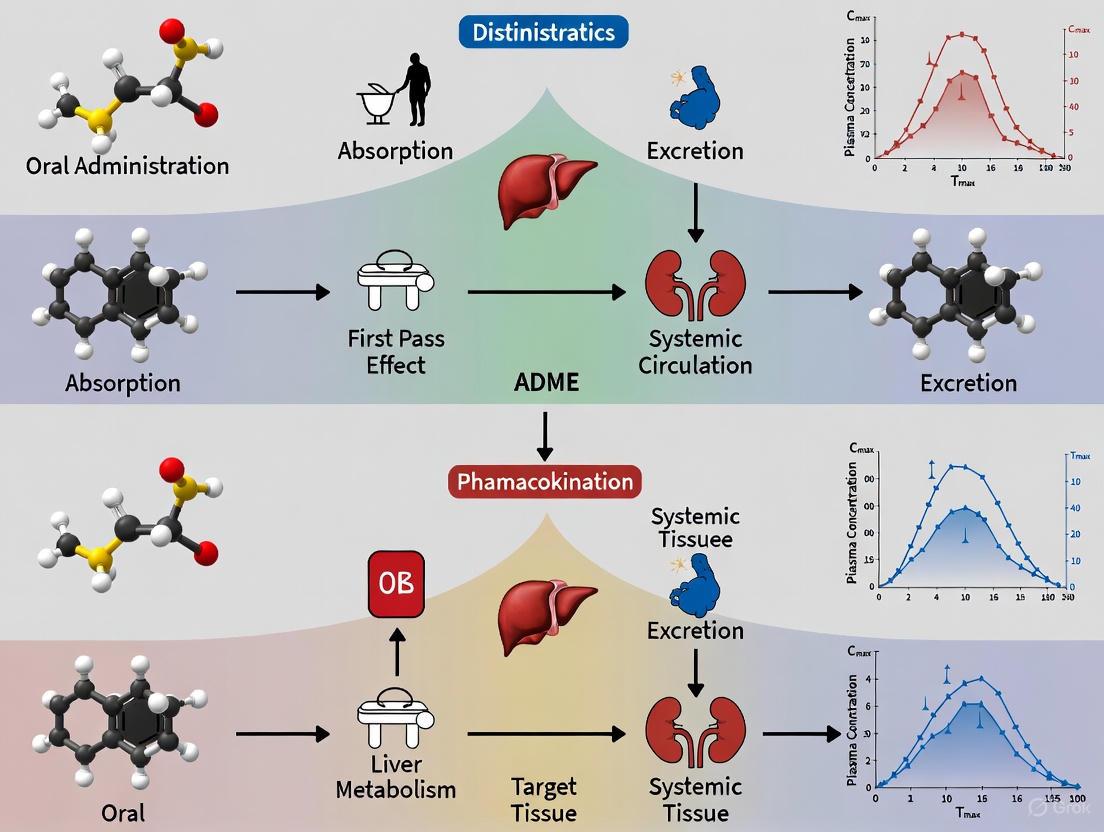
In the comparative pharmacokinetics of oral versus intravenous administration, a fundamental challenge has been obtaining intravenous pharmacokinetic (PK) data early in drug development. Traditional two-period crossover studies require extensive toxicology support and Good Manufacturing Practice (GMP)-quality IV formulations, making them resource-intensive. The microtracer approach revolutionizes this paradigm by enabling the simultaneous assessment of absolute bioavailability and IV PK using a minimally toxic, sub-therapeutic IV microtracer dose administered concomitantly with a therapeutic oral dose. This method provides critical IV pharmacokinetic parameters without standalone IV toxicology studies, accelerating early clinical development while maintaining scientific rigor and regulatory compliance.
Understanding a drug's absolute oral bioavailability (F) is crucial for development decisions, formulation optimization, and understanding exposure-response relationships. Absolute bioavailability is calculated by comparing systemic exposure after oral administration to exposure after IV administration: F = (AUCoral × DoseIV) / (AUCIV × Doseoral). Traditionally, this required separate clinical studies with full therapeutic IV doses, necessitating:
- Comprehensive IV toxicology studies in animals
- GMP-quality IV formulation development
- Two-period crossover designs with potential temporal effects
The microtracer approach circumvents these hurdles through the co-administration of a therapeutic oral dose with a minuscule IV dose containing a radiolabeled (¹â´C) or stable isotope (¹³C) version of the drug. This design captures both oral and IV PK from the same plasma samples at the same time, eliminating period effects while providing complete IV PK characterization with minimal radioactive exposure and regulatory burden.
Microtracer Methodology: Technical Implementation
Core Experimental Design
The microtracer approach employs an open-label, single-period design where subjects receive:
- A therapeutic oral dose of the non-labeled drug
- A simultaneous IV microtracer containing 100-1000 nCi of ¹â´C-labeled drug or stable isotope-labeled version
The IV microtracer dose is typically ≤1% of the oral therapeutic dose, well below pharmacological activity thresholds, which exempts it from requiring standalone IV toxicology studies. This design is frequently incorporated into Phase I SAD/MAD programs or human Absorption, Distribution, Metabolism, and Excretion (hADME) studies, leveraging existing clinical infrastructure for cost efficiency [40].
Analytical Methods
Accelerator Mass Spectrometry (AMS)
AMS provides the extreme sensitivity required to measure the minimal radioactivity from microtracer doses, capable of detecting one labeled drug molecule in 10¹²-10¹ⵠunlabeled molecules. For ¹â´C-labeled microtracers, AMS quantifies:
- Total drug-related material (total radioactivity) in plasma
- Parent drug concentrations after chromatographic separation
- Metabolite profiling in plasma and excreta [41] [42]
Liquid Chromatography-Mass Spectrometry (LC-MS/MS)
For stable isotope-labeled microtracers (e.g., ¹³C), LC-MS/MS differentiates between oral and IV drug based on mass differences, enabling simultaneous quantification of both administrations from single plasma samples [43].
Regulatory and Safety Considerations
Microtracer studies operate under specific regulatory provisions:
- Radiation exposure from ≤1 μCi (37 kBq) of ¹â´C is considered trivial risk (Category I, ICRP)
- Non-GMP ¹â´C-labeled drug may be acceptable with proper risk assessment
- Standard Phase I protocols apply with standard safety monitoring [42]
Table 1: Key Microtracer Parameters and Typical Values
| Parameter | Traditional IV Dosing | Microtracer Approach | Advantage |
|---|---|---|---|
| IV Dose Level | Therapeutic (mg range) | Microtracer (μg range) | Minimal safety risk |
| Toxicology Requirements | Full IV toxicology package | Often exempt | Reduced animal use, cost, time |
| IV Formulation | GMP required | May use well-characterized non-GMP | Development flexibility |
| Study Design | Two-period crossover | Single period | No temporal effects |
| Radioactivity | High (≥100 μCi) | Low (≤1 μCi) | Reduced subject burden |
| Bioanalysis | Standard LC-MS/MS | AMS or high-sensitivity LC-MS/MS | Ultra-sensitive detection |
Comparative Data: Microtracer vs. Traditional Approaches
Case Studies
Zabedosertib (IRAK4 Inhibitor)
A combined food-effect and absolute bioavailability study used a ¹³C-microtracer approach to determine an absolute oral bioavailability of 74% at 120 mg. The IV microtracer was administered concomitantly with the oral dose, providing simultaneous IV and oral PK data without a separate IV toxicology package [43].
Ipatasertib (Akt Inhibitor)
An open-label study assessed absolute bioavailability using an IV microtracer dose concomitant with an oral therapeutic dose. The approach successfully characterized ipatasertib's bioavailability and disposition while minimizing development resources [44].
Berzosertib (ATR Inhibitor)
A Phase 1 mass balance study incorporated a 210 mg/m² IV microtracer dose containing ~3 μCi of [¹â´C]berzosertib. The study established the predominant role of metabolic clearance without requiring standalone IV toxicology [41].
Iclepertin (GlyT1 Inhibitor)
PBPK model development incorporated data from a microtracer absolute bioavailability study (Study 1346.15), which provided critical IV PK parameters for model qualification without a traditional IV toxicology package [45].
Table 2: Application Examples of Microtracer Approach in Drug Development
| Drug | Therapeutic Class | Microtracer Type | Key Outcomes | Study Design |
|---|---|---|---|---|
| Zabedosertib | IRAK4 inhibitor | ¹³C-labeled | 74% absolute bioavailability | Combined with food-effect study |
| Ipatasertib | Akt inhibitor | ¹â´C-labeled | Mass balance and bioavailability | Two-period design |
| Berzosertib | ATR inhibitor | ¹â´C-labeled | 73.7% fecal excretion | Mass balance study |
| Iclepertin | GlyT1 inhibitor | ¹â´C-labeled | 72% absolute bioavailability | PBPK model qualification |
Comparative Advantages
The microtracer approach demonstrates clear advantages over traditional methods:
- Timeline Reduction: Eliminates 6-12 months for IV formulation and toxicology
- Cost Efficiency: ~70% cost reduction compared to traditional two-period design
- Scientific Superiority: Eliminates period effects through simultaneous administration
- Risk Mitigation: Provides early human IV PK to de-risk development decisions [40]
Experimental Protocols
Standard Microtracer Protocol
A typical microtracer study follows this workflow:
Key Methodological Details
Dose Preparation
- IV microtracer: ¹â´C-labeled drug with ~100-1000 nCi radioactivity in sterile solution
- Oral dose: Therapeutic dose of non-labeled drug in standard formulation
- Dose proportionality: Microtracer ≤1% of oral dose to maintain minimal exposure
Sample Collection and Analysis
- Blood sampling: Pre-dose and multiple post-dose timepoints (e.g., 0.5, 1, 2, 4, 8, 12, 24, 48, 72+ hours)
- Plasma separation: Immediate processing and storage at -70°C
- AMS analysis: For ¹â´C studies, measures total radioactivity and parent drug after separation
- LC-MS/MS analysis: For stable isotope studies, differentiates oral vs. IV drug by mass
The Scientist's Toolkit: Essential Research Reagents
Table 3: Essential Materials and Reagents for Microtracer Studies
| Item | Function | Technical Specifications |
|---|---|---|
| ¹â´C-Labeled Drug | IV microtracer component | Specific activity: 4.77 mCi/mmol (e.g., ipatasertib) [44]; Radioactivity: 3-37 kBq per dose |
| Stable Isotope-Labeled Drug | IV microtracer (alternative) | Isotopes: ¹³C, ²H, ¹âµN; Isotopic purity: >99%; Chemical purity: >95% |
| Accelerator Mass Spectrometer | Ultra-sensitive radioactivity detection | Sensitivity: 10â»Â¹âµ to 10â»Â¹â¸ for ¹â´C/¹²C ratios; Sample throughput: 10²-10³ samples/day |
| Liquid Scintillation Counter | Radioactivity quantification in excreta | Detection efficiency: >90% for ¹â´C; Background: <30 CPM |
| High-Resolution Mass Spectrometer | Metabolite identification | Resolution: >30,000; Mass accuracy: <5 ppm |
| Rapid Equilibrium Dialysis Device | Protein binding assessment | Membrane molecular weight cutoff: 10-15 kDa; Incubation time: 4-6 hours |
| Huperzine A | Huperzine A, MF:C15H18N2O, MW:242.32 g/mol | Chemical Reagent |
| Harpagide | Harpagide | High-purity Harpagide, an iridoid glycoside with anti-inflammatory and antiparasitic research applications. For Research Use Only. Not for human or veterinary use. |
The microtracer approach represents a paradigm shift in obtaining IV PK data during early drug development. By enabling the determination of absolute bioavailability and complete IV pharmacokinetic parameters without standalone IV toxicology, this methodology addresses a critical challenge in comparative oral vs. IV pharmacokinetics. The approach offers substantial advantages in development timeline, cost efficiency, and scientific rigor, making it an indispensable tool for modern drug development programs. As the case studies demonstrate, microtracer methodologies have been successfully applied across diverse therapeutic areas, providing critical human PK data to inform development decisions while maintaining the highest standards of subject safety and scientific validity.
Designing Bioavailability and Bioequivalence Studies for Regulatory Submission
Bioavailability (BA) and Bioequivalence (BE) studies form the cornerstone of pharmaceutical regulatory science, providing critical data that bridges drug development and therapeutic application. Within comparative pharmacokinetics research, these studies are particularly crucial for understanding the fundamental relationship between oral and intravenous administration—a comparison that establishes absolute bioavailability and informs rational dosing decisions across administration routes. The design and execution of these studies directly impact regulatory success, making strategic planning essential for obtaining market approval for new chemical entities, generic drugs, and approved products with modified routes of administration.
For researchers and drug development professionals, optimizing BA/BE study design requires meticulous attention to pharmacokinetic principles, regulatory requirements, and practical methodological considerations. This guide provides a comprehensive comparison of methodological approaches and experimental protocols, supported by current research data and structured to facilitate regulatory submission planning.
Key Pharmacokinetic Parameters in BA/BE Assessment
Table 1: Core Pharmacokinetic Parameters for BA/BE Studies
| Parameter | Definition | IV Route Reference | Oral Route Assessment | Regulatory Significance |
|---|---|---|---|---|
| AUC (Area Under the Curve) | Total drug exposure over time | Complete systemic exposure (AUCIV) | Fraction of dose reaching systemic circulation (AUCoral) | Primary endpoint for extent of absorption |
| Cmax | Maximum plasma concentration | Direct measurement of peak concentration | Influenced by absorption rate and extent | Key endpoint for rate of absorption |
| Tmax | Time to reach Cmax | Typically immediate post-injection | Determined by absorption kinetics | Supportive evidence of absorption rate |
| t1/2 (Half-life) | Time for plasma concentration to reduce by half | Independent of administration route | Should match IV half-life | Confirms similar elimination kinetics |
| F (Absolute Bioavailability) | Fraction of dose reaching systemic circulation | Reference value: 100% | F = (AUCoral × DoseIV) / (AUCIV × Doseoral) | Critical for route evaluation and dosing |
The fundamental parameters listed in Table 1 provide the quantitative foundation for comparing drug performance across administration routes. Absolute bioavailability (F) is particularly crucial when bridging intravenous and oral administration, as it quantifies the fraction of an orally administered dose that reaches systemic circulation unchanged [46]. Recent research emphasizes that understanding these parameters for both parent compounds and their metabolites is increasingly important, especially for drugs with active metabolites like tramadol, where route-dependent differences in metabolite exposure can occur [46].
Comparative Pharmacokinetics: Oral vs. Intravenous Administration
Quantitative Bioavailability Comparisons Across Drug Classes
Table 2: Bioavailability Comparison Across Drug Classes
| Drug/Drug Class | Absolute Oral Bioavailability | Key PK Differences (Oral vs. IV) | Clinical Implications | Study Evidence |
|---|---|---|---|---|
| Ibuprofen | 91% [46] | Equivalent AUC; delayed Tmax | Oral provides equivalent exposure to IV | Randomized clinical trial (2025) [46] |
| Tramadol | 80% [46] | Higher M1 metabolite exposure with oral route | IV produces higher parent drug bioavailability | Randomized clinical trial (2025) [46] |
| Ceftriaxone | SC: 99% (vs IV) [47] | Equivalent AUC and trough concentrations | Alternative administration routes possible | PhASAge Study (2025) in older adults [47] |
| Cephalexin | Varies (dose-dependent) [48] | Absorption influenced by maturational changes | Pediatric dosing requires adjustment | Population PK study in infants (2025) [48] |
| High Bioavailability Antibiotics (>90%) [49] | Fluconazole, Metronidazole, Clindamycin | Nearly equivalent exposure | Direct IV to oral switch feasible | Therapeutic guidelines [49] |
| Moderate Bioavailability Antibiotics (50-90%) [49] | Amoxicillin, Flucloxacillin, Ciprofloxacin | Moderate reduction in exposure | May require dose adjustment when switching | Therapeutic guidelines [49] |
The data in Table 2 demonstrates significant variability in how different drugs and drug classes behave when administered via different routes. For some medications like ibuprofen, oral administration can achieve bioavailability exceeding 90%, suggesting minimal first-pass metabolism and excellent absorption [46]. In contrast, other drugs exhibit more complex pharmacokinetics where route administration significantly influences not just parent drug exposure but also metabolite profiles, as evidenced by tramadol's route-dependent differences in its active O-desmethyl metabolite (M1) formation [46].
Clinical Outcomes and Therapeutic Equivalence
Beyond pharmacokinetic parameters, clinical outcomes provide critical evidence supporting route equivalence or superiority. Recent research has challenged historical preferences for intravenous administration across numerous infection types. Multiple randomized controlled trials have demonstrated equivalent efficacy between oral and intravenous antibiotics for conditions including pneumonia, urinary tract infections, intra-abdominal infections, and even serious infections like bacteremia and endocarditis in stable patients [50]. The Oral versus Intravenous Antibiotics for Bone and Joint Infection (OVIVA) trial found early switching to oral therapy non-inferior to continued IV treatment for bone and joint infections [49] [50].
For patients, oral administration typically offers improved quality of life, reduced healthcare utilization, and lower risk of device-related complications [49] [50]. These clinical findings underscore the importance of well-designed BA/BE studies in challenging therapeutic dogmas and expanding appropriate use of oral formulations.
Methodological Framework for BA/BE Study Design
Core Study Design Elements
BA/BE Study Workflow
The diagram above outlines the standardized workflow for a typical BA/BE study, incorporating key design elements such as randomization, crossover, and adequate washout periods. The critical serial blood sampling scheme is expanded to show typical timepoints needed to properly characterize the concentration-time profile for both intravenous and oral administration.
Standardized Experimental Protocols
Clinical Study Protocol Template
Study Design: Randomized, open-label, single-dose, two-period, two-sequence crossover design is preferred for BA/BE studies [46]. This design efficiently controls for inter-subject variability by having each subject serve as their own control.
Subject Population: Healthy volunteers (unless safety concerns preclude), with sample size sufficient to provide adequate statistical power (typically 12-36 subjects) [46]. Recent studies emphasize inclusion of demographic subsets (e.g., by sex, age) when relevant to the drug's use population [46].
Dosing Conditions: Standardized fasting/fed conditions consistent with the drug's intended use. For oral-IV comparisons, the IV dose may be lower than oral to achieve similar exposure while ensuring accurate analytical measurement [46].
Blood Sampling Schedule: Intensive sampling around expected Tmax with continued sampling through at least 3-5 terminal half-lives. Specific sampling timepoints should be justified based on known pharmacokinetics [46] [48].
Analytical Methods: Validated bioanalytical methods (typically LC-MS/MS) with demonstrated specificity, accuracy, precision, and sensitivity appropriate for expected concentration ranges. For chiral compounds like ibuprofen, enantiomer-specific methods may be required [46].
Bioanalytical Method Validation
A comprehensive validation should establish:
- Selectivity/Specificity: No interference from endogenous compounds
- Linearity: Calibration curve with correlation coefficient (r²) >0.99
- Accuracy and Precision: Within ±15% for all quality controls (±20% at LLOQ)
- Stability: Under storage, processing, and injection conditions
- Recovery: Consistent and reproducible extraction efficiency
Statistical Approaches for BA/BE Assessment
Acceptance Criteria for Bioequivalence
Regulatory agencies worldwide have established standardized statistical criteria for demonstrating bioequivalence:
Primary Endpoints: AUC0-t, AUC0-∞, and Cmax are typically the primary endpoints for BE determination.
Statistical Analysis: Logarithmically transformed parameters are analyzed using ANOVA.
Equivalence Range: The 90% confidence interval for the geometric mean ratio (Test/Reference) must fall entirely within 80.00-125.00% for AUC parameters and Cmax [46].
Recent research has highlighted situations where these standard criteria may require refinement. For instance, drugs with complex pharmacokinetics such as tramadol may demonstrate bioequivalence for the parent drug while showing differences in metabolite profiles that could potentially impact clinical outcomes [46].
Special Considerations for Oral vs. IV Comparisons
When comparing oral to intravenous administration specifically, several unique factors require consideration:
Dose Selection: Due to complete bioavailability of IV administration, doses are often unequal, requiring dose-normalization of pharmacokinetic parameters [46].
Absolute Bioavailability Calculation: F = (AUCoral × DoseIV) / (AUCIV × Doseoral) × 100%
Metabolite Considerations: As seen with tramadol, IV administration may produce higher parent drug bioavailability but lower active metabolite exposure compared to oral route [46].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Essential Research Reagents and Materials for BA/BE Studies
| Category | Specific Items | Function & Application | Technical Considerations |
|---|---|---|---|
| Reference Standards | • Drug substance (parent compound) |
Quantification of analytes in biological matrices; method development and validation | >95% purity; proper storage conditions; certificate of analysis required [46] |
| Bioanalytical Instruments | • LC-MS/MS system• HPLC with UV/fluorescence detection• Automated sample preparation systems | Separation, detection, and quantification of analytes in biological samples | Validation required per FDA/EMA guidelines; appropriate sensitivity for expected concentrations [46] |
| Biological Matrices | • Human plasma/serum• Blank matrices from appropriate species• Quality control materials | Study samples; method development; quality controls | Proper collection, processing, and storage conditions to maintain stability [48] |
| Clinical Supplies | • Clinical trial material (test and reference)• Matching placebo• Administration supplies (syringes, catheters, etc.) | Dosing in clinical phase; blinding requirements | GMP-manufactured; stability data; proper blinding procedures [47] |
| Specialized Reagents | • Chiral columns (for enantiomer separation)• Protein precipitation reagents• Solid-phase extraction cartridges | Specialized analytical needs; sample clean-up | Method-specific optimization required; validation of enantiomer-specific methods [46] |
| Streptozocin | Streptozocin | Bench Chemicals | |
| Patulin | Patulin Mycotoxin Reference Standard | High-purity Patulin for food safety and toxicology research. This product is For Research Use Only (RUO). Not for diagnostic, therapeutic, or personal use. | Bench Chemicals |
The reagents and materials listed in Table 3 represent the essential toolkit for executing successful BA/BE studies. Recent methodological advances have emphasized the importance of enantiomer-specific analysis for chiral drugs like ibuprofen, where the two enantiomers may exhibit different pharmacokinetic and pharmacodynamic properties [46]. Additionally, population pharmacokinetic approaches are increasingly valuable, particularly for special populations like infants where maturational changes significantly impact drug absorption and clearance [48].
Regulatory Strategy and Submission Framework
Regulatory Submission Pathways
Table 4: Key Regulatory Submission Types for BA/BE Data
| Submission Type | Purpose | BA/BE Data Requirements | Authority |
|---|---|---|---|
| IND (Investigational New Drug) | Initiate clinical trials in humans | Preliminary BA data; supporting safe starting doses | FDA [51] |
| NDA (New Drug Application) | Market approval for new drug | Comprehensive BA studies; definitive BA profile | FDA [51] |
| ANDA (Abbreviated NDA) | Market approval for generic drug | BE studies vs. reference listed drug | FDA [51] |
| BLA (Biologics License Application) | Market approval for biologics | BA data for relevant routes; comparative PK | FDA [51] |
| MAA (Marketing Authorization Application) | Market approval in EU | BA/BE data per EMA guidelines | EMA [51] |
Accelerating Regulatory Submissions
Leading pharmaceutical companies are implementing innovative approaches to compress regulatory submission timelines, potentially reducing filing periods from historical averages of 6-9 months to 8-12 weeks in some cases [52]. Key strategies include:
Simplified Filing Strategy: Developing a filing strategy focused on rigorously defining and targeting the desired product label, ensuring clinical programs efficiently demonstrate the product's benefit-risk profile [52].
Zero-Based Redesign of Submission Process: Fundamentally redesigning the submission process from the last patient's last visit through to filing, incorporating parallel processing of activities and removing non-essential dossier activities [52].
Technology Enablement: Implementing modern regulatory-information-management systems (RIMS) that enable seamless workflows, embedded automation, and data-centric approaches [52].
AI-Enhanced Content Generation: Utilizing generative AI for medical writing, which early pilots suggest can reduce end-to-end cycling time for authoring clinical-study reports by 40% [52].
Well-designed bioavailability and bioequivalence studies provide essential data that bridges preclinical development and clinical application, particularly when comparing administration routes. The comparative pharmacokinetics of oral versus intravenous administration continues to reveal important insights that challenge historical therapeutic dogmas and expand appropriate use of oral formulations across numerous drug classes and clinical conditions.
As regulatory science evolves, successful drug development programs will increasingly integrate BA/BE considerations early in development, leverage technological advances in bioanalysis and data submission, and implement strategic regulatory approaches that accelerate approval timelines while maintaining rigorous standards for demonstrating therapeutic equivalence.
For orally administered drugs, the consumption of food can significantly alter their absorption profile, a phenomenon known as the "food effect" that impacts approximately 40% of orally administered drugs [53]. Understanding and predicting these effects is crucial for developing appropriate administration guidelines in drug labeling. Traditionally, food effect assessment has relied heavily on clinical studies, which require significant investment of time and resources [54]. This case study explores the application of Physiologically-Based Pharmacokinetic (PBPK) modeling as a powerful mechanistic tool to predict food effects on drug absorption, comparing its performance and utility against traditional clinical studies and other modeling approaches.
PBPK modeling integrates drug-specific properties with organism-specific physiological parameters to simulate drug concentrations in various tissues and organs over time [55]. Unlike classical compartmental PK methods, PBPK models employ a "bottom-up" approach, building simulations from fundamental physicochemical and physiological principles [55]. This mechanistic framework is particularly valuable for predicting food effects, as it can incorporate the complex changes in gastrointestinal physiology that occur after meal consumption, including altered gastric emptying, bile salt secretion, luminal pH, and blood flow [54].
Theoretical Foundation of PBPK Modeling for Food Effects
Fundamental Principles of PBPK Modeling
PBPK modeling represents a paradigm shift from traditional top-down pharmacokinetic approaches. Rather than simply fitting curves to observed plasma concentration data, PBPK models mechanistically simulate drug disposition by representing the body as an interconnected network of physiologically relevant compartments [56]. Each compartment corresponds to a specific organ or tissue (e.g., liver, gut, kidney) characterized by its volume, blood flow, and tissue composition [55] [56]. These models integrate three primary parameter types: (1) organism-specific parameters (organ volumes, blood flows, protein levels), (2) drug-specific parameters (lipophilicity, solubility, pKa, permeability), and (3) drug-biological system interaction parameters (tissue-plasma partition coefficients, fraction unbound) [55] [56].
For food effect prediction, PBPK models specifically incorporate the physiological changes induced by food intake, including increased gastric and intestinal fluid volumes, altered motility patterns, secretion of bile salts, and changes in luminal pH [53] [54]. These modifications allow the model to simulate how the dissolution, solubility, and permeability of a drug might be affected in the fed versus fasted state.
Comparison of PBPK with Alternative Modeling Approaches
PBPK modeling occupies a distinct position in the spectrum of pharmacokinetic modeling techniques, particularly when compared to Population PK (PopPK) modeling.
Table 1: Comparison of PBPK and PopPK Modeling Approaches
| Feature | PBPK Modeling | Population PK (PopPK) Modeling |
|---|---|---|
| Fundamental Approach | Bottom-up (mechanistic) | Top-down (empirical) |
| Compartments | Represent specific organs/tissues | Lack direct physiological correspondence |
| Parameter Source | In vitro data, physicochemical properties | Observed clinical PK data |
| Variability Assessment | Typically describes average subject | Estimates inter-individual variability |
| Key Applications | Food effect prediction, DDI assessment, special populations | Identifying sources of variability, dosing optimization |
| Data Requirements | Extensive preclinical compound data | Rich clinical PK datasets |
While PopPK models are valuable for identifying sources of variability in clinical data, PBPK models offer superior mechanistic insight and predictive capability for food effects, especially when clinical data are limited [57]. The bottom-up nature of PBPK allows for prospective prediction of food effects before clinical studies are conducted, enabling more efficient drug development.
Case Study: PBPK-Based Food Effect Prediction Workflow
Middle-Out Modeling Strategy
Successful application of PBPK for food effect prediction typically employs a "middle-out" approach, which integrates both bottom-up and top-down methodologies [55] [54]. This strategy begins with developing and verifying a base PBPK model using clinical data from one prandial state (usually fasted). The verified model then simulates drug pharmacokinetics under the alternative prandial condition (fed state), and these predictions are compared against clinical data to evaluate predictive performance [54]. This hybrid approach leverages both mechanistic understanding and empirical validation, balancing scientific rigor with practical utility.
Experimental Data Requirements and Input Parameters
Building a reliable PBPK model for food effect prediction requires comprehensive compound-specific data, which can be categorized into several key domains:
- Physicochemical Properties: Lipophilicity (LogP/LogD), pKa values, molecular weight, solubility profile across physiological pH range [56] [54].
- Biopharmaceutical Properties: Permeability (e.g., Caco-2, PAMPA), dissolution characteristics, biorelevant solubility in fasted and fed state simulated intestinal fluids (FaSSIF/FeSSIF) [53] [54].
- Metabolic and Transport Properties: Metabolic stability, enzyme kinetics, transporter affinities [55] [56].
These parameters are typically obtained through standardized in vitro assays and incorporated into the PBPK platform to establish the base model.
Workflow Diagram: PBPK Food Effect Prediction
The following diagram illustrates the systematic workflow for applying PBPK modeling to predict food effects:
Performance Assessment: PBPK vs. Traditional Methods
Quantitative Prediction Accuracy
The predictive performance of PBPK modeling for food effects has been systematically evaluated across multiple compounds. A comprehensive assessment by the Food Effect PBPK IQ Working Group analyzed 30 compounds using standardized methodology and decision trees for model verification [53]. The results demonstrate promising predictive capability:
Table 2: PBPK Food Effect Prediction Accuracy Across 30 Compounds
| Prediction Confidence Level | Number of Compounds | Prediction Accuracy Range (fold) | Success Rate |
|---|---|---|---|
| High Confidence | 15 | 0.8- to 1.25-fold | 50% |
| Moderate Confidence | 8 | 0.5- to 2.0-fold | 27% |
| Low Confidence | 7 | >2.0-fold | 23% |
This evaluation found no clear difference in prediction success for positive versus negative food effects, nor a definitive relationship to the Biopharmaceutics Classification System (BCS) category of the tested molecules [53]. However, prediction success was particularly strong when the food effect mechanism primarily involved changes in gastrointestinal luminal conditions, including fluid volume, motility, pH, micellar entrapment, and bile salt concentrations [53].
Comparative Analysis with Clinical Studies
Traditional food effect assessment requires dedicated clinical trials, typically employing a standardized high-fat meal and crossover design with pharmacokinetic sampling [58] [54]. The following table compares key aspects of both approaches:
Table 3: PBPK Modeling vs. Traditional Clinical Studies for Food Effect Assessment
| Characteristic | PBPK Modeling | Traditional Clinical Study |
|---|---|---|
| Time Requirements | Weeks to months | Several months (planning, ethics, conduct, analysis) |
| Cost Implications | Moderate (primarily personnel and software) | High (clinical operations, subject compensation, analytics) |
| Mechanistic Insight | High (isolates contributing factors) | Limited (provides net effect without mechanism) |
| Regulatory Acceptance | Growing (case-by-case basis) | Standard requirement |
| Predictive Capability | Prospective (before clinical data) | Retrospective (requires human data) |
| Population Extrapolation | Flexible (special populations, pediatrics) | Limited to studied population |
Clinical studies, such as the one investigating the CDK4/6 inhibitor SHR6390, demonstrate the magnitude of food effects that PBPK models aim to predict. In that study, a high-fat meal increased the maximum plasma concentration (Cmax) by 56.9% and the area under the curve (AUC) by approximately 38% compared to the fasting state [58].
Research Toolkit for PBPK Food Effect Modeling
Implementing PBPK modeling for food effect prediction requires specific computational and experimental resources. The following toolkit outlines essential components:
Table 4: Research Toolkit for PBPK Food Effect Modeling
| Tool Category | Specific Tools/Assays | Function in PBPK Workflow |
|---|---|---|
| PBPK Software Platforms | GastroPlus, Simcyp Simulator, PK-Sim | Provide physiological framework, database, and simulation algorithms |
| In Vitro Assay Systems | Caco-2 permeability, biorelevant dissolution, FaSSIF/FeSSIF solubility | Quantify key input parameters for absorption modeling |
| Analytical Instruments | LC-MS/MS systems | Determine plasma concentrations for model verification |
| Physicochemical Characterization | pKa analyzers, logP determination, solubility profiling | Define fundamental drug properties |
| Clinical Data | Fasted and fed state PK studies | Model verification and validation |
| Erythromycin Ethylsuccinate | Erythromycin Ethylsuccinate, CAS:41342-53-4, MF:C43H75NO16, MW:862.1 g/mol | Chemical Reagent |
| Diacerein | Diacerein|C19H12O8|IL-1 Inhibitor For Research | High-purity Diacerein, a potent interleukin-1 (IL-1) inhibitor for osteoarthritis and inflammation research. For Research Use Only. Not for human use. |
Commercial PBPK platforms such as GastroPlus and Simcyp Simulator include extensive physiological libraries and specialized modules for food effect simulation, incorporating systems data on how food intake alters gastrointestinal physiology [55] [54]. These platforms have been instrumental in advancing the field by providing standardized frameworks for food effect prediction.
Regulatory Considerations and Future Directions
Regulatory agencies increasingly recognize the value of PBPK modeling in drug development. The U.S. Food and Drug Administration (FDA) has utilized PBPK models to evaluate various aspects of drug product performance, including the impact of food on bioavailability [38]. A well-established and validated PBPK absorption model can support regulatory decision-making by providing a robust framework for justifying bioequivalence study designs and assessing risks associated with formulation changes [38].
Future advancements in PBPK modeling for food effects will likely focus on enhancing predictive confidence for complex scenarios, such as drugs with transporter-mediated absorption or those with complex dissolution profiles. Additionally, developing patient-centric dissolution quality standards using PBPK modeling that account for food effects across different disease states remains an area of active research [38]. Continued collaboration between industry, academia, and regulators will be essential to establish best practices and increase regulatory acceptance of PBPK-based food effect predictions.
This case study demonstrates that PBPK modeling provides a powerful, mechanistic approach for predicting food effects on drug absorption. When appropriately verified, PBPK models can predict food effects with high to moderate confidence for a substantial proportion of compounds, potentially reducing the need for dedicated clinical studies in some cases [53] [54]. The middle-out approach, which combines bottom-up mechanistic modeling with targeted clinical verification, offers a scientifically rigorous and resource-efficient strategy for food effect assessment.
While traditional clinical studies remain the regulatory standard, PBPK modeling serves as a valuable complementary tool that provides deeper mechanistic insight and enables prospective prediction. As the science continues to evolve and best practices become established, PBPK modeling is positioned to play an increasingly important role in streamlining oral drug product development and optimizing dosing recommendations for administration in relation to meals.
Establishing a safe and effective initial human dose is a critical milestone in drug development, bridging preclinical research and clinical trials. This process relies heavily on pharmacokinetic (PK) parameters—the quantitative measures of how the body handles a drug—to extrapolate animal data to human predictions. Within the broader context of comparative pharmacokinetics, the route of administration, particularly the fundamental differences between oral (PO) and intravenous (IV) administration, profoundly influences these PK parameters and the subsequent dose prediction strategy [22].
IV administration delivers a drug directly into the bloodstream, resulting in 100% bioavailability and immediate onset of action, while oral administration introduces variables of absorption and a first-pass metabolism effect that can significantly reduce the systemic availability of the drug [22]. Understanding these differences is not merely academic; it directly informs which PK parameters are used in allometric scaling and how predictive models are constructed. This guide objectively compares the methodologies, experimental data, and practical applications of these approaches, providing a framework for researchers to translate PK data into the first safe doses for human trials.
Core PK Parameters and the Influence of Administration Route
The journey from data to dosing hinges on accurately measuring and interpreting key PK parameters. These parameters describe a drug's absorption, distribution, metabolism, and excretion (ADME). The route of administration fundamentally shapes the profile of these parameters.
- Absorption: For oral drugs, bioavailability (F) is a critical parameter, defined as the fraction of an administered dose that reaches the systemic circulation unchanged. It is influenced by formulation, gastrointestinal stability, and intestinal permeability. IV drugs, by definition, have a bioavailability of 100% [22].
- Distribution: The volume of distribution (Vd) estimates the theoretical space in the body into which a drug has distributed. A high Vd often suggests extensive tissue binding, while a low Vd suggests the drug is largely confined to the plasma.
- Clearance and Elimination: Clearance (CL) is the volume of plasma from which a drug is completely removed per unit time. It is a key determinant of drug exposure. The elimination half-life (t½) describes the time required for the plasma concentration to reduce by half and is dependent on both Vd and CL.
The following table summarizes how the administration route impacts these core processes.
Table 1: Impact of Administration Route on Key Pharmacokinetic Parameters
| PK Process | Intravenous (IV) | Oral (PO) |
|---|---|---|
| Absorption | Immediate and complete (100% bioavailability) [22] | Delayed and often incomplete due to GI absorption and first-pass metabolism [22] |
| First-Pass Effect | Bypassed [22] | Significant; drug passes through liver via portal circulation before reaching systemic circulation, potentially reducing active drug concentration [22] |
| Onset of Action | Rapid [22] | Slower |
| Peak Concentration | Higher and reached quickly | Lower and reached later |
| Control over Drug Levels | Precise | Less precise |
A practical example of these differences was demonstrated in a study of chloramphenicol in infants with Haemophilus influenzae meningitis. Switching from IV to oral administration at the same dose resulted in a longer mean serum half-life (increasing from 4.0 to 6.5 hours) and higher trough CSF levels with the oral formulation, occasionally leading to drug accumulation [19]. This underscores that a simple mg-for-mg switch between routes is not always appropriate and highlights the need for route-specific PK analysis.
Methodologies for Predicting Human PK Parameters and FIH Dose
Allometric Scaling from Preclinical Species
Allometric scaling is a widely used empirical method for predicting human PK parameters by extrapolating data from animal studies based on body weight and physiological similarities across species [59] [60]. The fundamental principle is expressed by the power equation: ( CL = a \times (BW)^b ), where CL is clearance, BW is body weight, and 'a' and 'b' are the allometric coefficient and exponent, respectively [60].
Several refined methods have been developed to improve the accuracy of simple allometric scaling:
- Rule of Exponents (ROE): This method uses brain weight (BrW) and maximum lifespan potential (MLP) to correct the scaling of clearance based on the value of the exponent 'b' [60].
- fu-Corrected Intercept Method (FCIM): This approach incorporates species differences in plasma protein binding (f~u~) to improve the prediction of human clearance [60].
- Two-Species Scaling (TS): Using data from rats and dogs, this method can provide accurate human clearance predictions with the formula: ( CL{human} = a{(rat-dog)} \times (BW_{human})^{0.628} ) [60].
Table 2: Human Clearance (CL) Prediction Methods Based on Allometric Scaling [60]
| Method | Description | Formula (mL/min) |
|---|---|---|
| SAS~N≥2~ | Simple allometric scaling using at least two species' data | CL = a × (BW)^b^ |
| ROE~N≥2~ | Rule of Exponents method using at least two species' data | If 0.71 < b ≤ 1, CL × MLP = a × (BW)^b^; If 1 < b ≤ 1.3, CL × BrW = a × (BW)^b^ |
| FCIM~R~ | Free drug fraction corrected method using rat data | CL~human~ = 33.35 × (a/R~fu~)^0.770^, R~fu~= f~uprat~/f~uphuman~ |
| TS~R,D~ | Two-species scaling using rat and dog data | CL~human~ = a~(rat-dog)~ × (BW~human~)^0.628^ |
In Vitro-In Vivo Extrapolation (IVIVE)
IVIVE uses in vitro assay data to predict in vivo human PK parameters. For instance, permeability data from cell models like Caco-2 can be used to estimate the human absorption rate constant (k~a~) and fraction absorbed (F~a~) [60]. Metabolic stability data from human liver microsomes or hepatocytes can be scaled using models like the well-stirred model to predict in vivo metabolic clearance [60].
Integrated Mechanistic Approaches
The trend in modern drug development is toward integrated, mechanistic approaches. For example, a study on a New Composition Bee Venom (NCBV) integrated PK data from three animal species using nonlinear mixed-effect modeling (NONMEM) to build a robust PK model [59]. This model was then used with allometric scaling (specifically the brain weight method) to predict human PK and back-calculate an initial FIH subcutaneous dose of 1.3 mg for a 70 kg human, based on the efficacious exposure in mice [59]. This showcases a hybrid strategy that leverages both empirical scaling and sophisticated modeling.
Experimental Protocols for Generating Key PK Data
Preclinical Pharmacokinetic Study Protocol
Objective: To characterize the ADME properties of a drug candidate in preclinical species to obtain data for allometric scaling.
Methodology:
- Animals and Dosing: Conduct PK studies in relevant species (e.g., mice, rats, dogs, non-human primates). Administer the drug via both IV and PO routes. The IV route provides fundamental parameters like clearance and V~d~ without the confounding variable of absorption [59].
- Sample Collection: Collect serial blood/plasma samples at predetermined time points after administration (e.g., pre-dose, 5, 15, 30 mins, 1, 2, 4, 8, 24 hours) [59].
- Bioanalysis: Process plasma samples and analyze drug concentrations using a validated analytical method, such as Liquid Chromatography with tandem mass spectrometry (LC-MS/MS) or Enzyme-Linked Immunosorbent Assay (ELISA) [59].
- Non-Compartmental Analysis (NCA): Use software like Phoenix WinNonlin to calculate primary PK parameters from the concentration-time data, including C~max~, T~max~, AUC~0-inf~, t~½~, V~d~, and CL [59].
- Protein Binding Assessment: Determine the fraction unbound (f~u~) in plasma across species using methods like equilibrium dialysis or ultracentrifugation, as this is critical for certain scaling methods [60].
Key Reagents and Materials for PK Studies
Table 3: Research Reagent Solutions for Pharmacokinetic Studies
| Item | Function/Description |
|---|---|
| LC-MS/MS System | Gold-standard analytical instrument for quantifying drug concentrations in biological matrices with high sensitivity and specificity. |
| Validated Bioanalytical Assay | A method meeting regulatory standards for accuracy, precision, and selectivity to ensure reliable concentration data. |
| Caco-2 Cell Line | A human colon adenocarcinoma cell line used in vitro to model drug permeability and absorption across the intestinal barrier [60]. |
| Human Liver Microsomes/Hepatocytes | In vitro systems used to study metabolic stability, metabolite identification, and to predict in vivo metabolic clearance via IVIVE [60]. |
| Equilibrium Dialysis Device | Used to determine the plasma protein binding (f~u~) of a drug candidate, a critical parameter for distribution and clearance predictions. |
| Phoenix WinNonlin Software | Industry-standard software for performing non-compartmental pharmacokinetic analysis of concentration-time data. |
| NONMEM Software | A powerful tool for nonlinear mixed-effects modeling, used for population PK analysis and building integrated PK models across species [59]. |
A Case Study: FIH Dose Prediction for a New Composition Bee Venom
A concrete example of the mechanistic approach is illustrated by the development of a New Composition Bee Venom (NCBV) for Alzheimer's disease [59]. The research team first conducted subcutaneous PK studies of NCBV in mice, rats, and beagle dogs. The individual animal data from these three species were integrated and analyzed using nonlinear mixed-effect modeling (NONMEM) to build a robust, population-based PK model [59].
This animal PK model was then used to define the PK parameters for each species. To scale these parameters to humans, three allometric scaling approaches were compared: simple, brain weight (BrW) incorporated, and maximum lifespan potential (MLP) incorporated. The BrW method showed the highest correlation (R² = 0.974) and was selected for the final prediction [59].
The FIH dose was calculated by determining the dose in a 70 kg human that would achieve the same area under the concentration-time curve (AUC = 0.397 μg·h/mL) observed after an efficacious dose of 0.1 mg/kg in mice. The predicted initial doses were 5.5 mg (simple), 1.3 mg (BrW), and 3.5 mg (MLP). The study ultimately recommended a subcutaneous FIH dose of 1.3 mg NCBV [59]. This dose was further supported by its alignment with the safe dose range (0.1 to 3 mg) suggested by the no observed adverse effect level (NOAEL) study, demonstrating a rational integration of efficacy and safety data [59].
Workflow Diagram: From Preclinical Data to First-In-Human Dose
The following diagram visualizes the workflow for establishing the first-in-human dose, integrating the methodologies and case study principles discussed.
Diagram Title: Workflow for First-in-Human Dose Estimation
Establishing the initial human dose is a multifaceted process that relies on the systematic generation of preclinical PK data and its intelligent extrapolation through validated methodologies. The comparative framework of IV vs. PO pharmacokinetics provides essential insights into how a drug's route of administration influences its PK profile and must be accounted for in predictive models.
As demonstrated, the field is moving beyond simple allometric scaling toward integrated, mechanistic approaches that combine in vitro data, in vivo PK from multiple species, and sophisticated modeling techniques like PBPK and NONMEM. The NCBV case study exemplifies this modern paradigm, where a hybrid strategy successfully bridged animal efficacy data to a rational, safe, and scientifically justified FIH dose. By adhering to these rigorous, data-driven principles, drug development professionals can de-risk early clinical trials and pave a more efficient path for bringing new therapeutics to patients.
Overcoming Oral Delivery Hurdles: Strategies for Bioavailability Enhancement and Variability Reduction
The oral route remains the most preferred and convenient pathway for drug administration due to its non-invasiveness, cost-effectiveness, and high patient compliance [61]. However, the therapeutic success of orally administered drugs is fundamentally dependent on their bioavailability, defined as the rate and extent at which the active pharmaceutical ingredient (API) becomes available at the site of action [61]. A drug's journey from ingestion to systemic circulation is fraught with challenges, the most significant being its aqueous solubility and intestinal permeability. According to the Biopharmaceutical Classification System (BCS), drugs are categorized into four classes based on these properties, with BCS Class II (low solubility, high permeability) and Class IV (low solubility, low permeability) posing the greatest formulation challenges [62] [61].
The pharmaceutical industry faces a critical hurdle: an estimated 40% of marketed oral drugs and up to 90% of new chemical entities (NCEs) are poorly water-soluble [63] [61]. This poor solubility often leads to inadequate and variable oral bioavailability, non-linear pharmacokinetics, and high food effects, ultimately diminishing therapeutic potential [62]. While intravenous administration guarantees 100% bioavailability by direct entry into systemic circulation, the development of advanced formulation strategies aims to narrow this bioavailability gap for oral dosage forms, making them therapeutically equivalent to their IV counterparts where clinically appropriate [64].
This guide objectively compares two leading technological approaches—nanocarrier systems and amorphous solid dispersions (ASDs)—for enhancing the solubility and bioavailability of poorly soluble drugs, providing experimental data and methodologies to inform rational formulation selection.
Formulation Strategy 1: Nanocarrier Systems
Nanocarriers are submicron-sized (typically 1-1000 nm) drug delivery vehicles engineered to encapsulate or dissolve poorly soluble APIs, thereby enhancing their aqueous solubility, protecting them from degradation, and facilitating targeted delivery [63] [65]. Their tiny size and large surface area-to-volume ratio are key to their performance.
Key Mechanisms of Action
- Solubilization Enhancement: Nanocarriers create a protected microenvironment for the drug, presenting it in a dissolved or amorphous state that is more readily absorbed [63].
- Bioavailability Improvement: By enhancing solubility and maintaining supersaturation, nanocarriers increase the concentration gradient across the intestinal lumen, driving passive diffusion and improving bioavailability [63] [66].
- Passive Targeting: The enhanced permeability and retention (EPR) effect, particularly for nanoemulsions and liposomes, allows selective accumulation in diseased tissues like tumors [63].
Types of Nanocarriers and Experimental Data
Table 1: Comparison of Major Nanocarrier Types for Solubility Enhancement
| Nanocarrier Type | Typical Composition | Average Size (nm) | Key Advantages | Reported Efficacy (from Search Results) |
|---|---|---|---|---|
| Lipid Nanoparticles (LNPs) | Ionizable lipids, DSPE-PEG, phospholipids [63] | 162-200 [63] | Low toxicity, biocompatible, scalable production [63] | siRNA encapsulation efficiency up to 98.8% [63] |
| Niosomes | Amphiphilic surfactants [63] | ~200 [63] | Can encapsulate both hydrophilic & lipophilic drugs [63] | Improved tumoricidal efficacy of methotrexate & doxorubicin [63] |
| Liposomes | DSPE-PEG/DSPG/HSPC phospholipids [63] | 100-120 [63] | High drug loading, flexible surface modification [63] | Optimized cisplatin loading at specific lipid ratios [63] |
| Polymeric Nanoparticles | HEC-PEI2k polymer [63] | 140-180 [63] | Controlled release, high stability [63] | >90% cell viability, high transfection efficiency [63] |
Critical Experimental Protocols
Objective: To determine the maximum drug solubility in pre-formed lipid nanocarriers and identify drug localization (core vs. interface).
- Preparation of Unloaded Nanocarriers: Prepare drug-free nanocarrier dispersions using high-pressure homogenization or microfluidization. Characterize the initial particle size, PDI, and zeta potential.
- Incubation with Drug: Incubate the unloaded nanocarriers with an excess of the crystalline drug substance under constant agitation for a sufficient time (typically 24-48 hours) to reach equilibrium.
- Separation: Remove undissolved drug crystals by filtration or centrifugation.
- Drug Quantification: Determine the amount of drug dissolved in the nanocarriers using UV spectroscopy or HPLC. The drug load is calculated as mass of drug per mass of lipid.
- Localization Assessment: Repeat the loading process with the same lipid matrix processed to different particle sizes. If the drug load remains constant regardless of particle size, the drug is predominantly in the core. If the drug load decreases with increasing particle size (and thus decreasing total surface area), the drug is substantially located at the lipid-water interface.
Objective: To ensure nanocarriers meet critical quality attributes for stability and performance.
- Particle Size & PDI: Use Dynamic Light Scattering (DLS). Samples must be diluted in a solvent of known viscosity. Measurements are sensitive to dust and aggregates.
- Surface Charge: Determine Zeta Potential via Laser Doppler Velipsometry. Sample dilution is required, and results are highly sensitive to ionic strength and pH.
- Morphology: Use Transmission Electron Microscopy (TEM) or Atomic Force Microscopy (AFM). These techniques provide direct visualization of particle shape and structure, confirming DLS data.
Formulation Strategy 2: Amorphous Solid Dispersions
Amorphous Solid Dispersions (ASDs) represent a powerful formulation approach where a poorly soluble crystalline API is dispersed within a hydrophilic polymeric carrier in an amorphous state. This conversion from a crystalline to a higher-energy amorphous state is a primary driver for enhanced solubility and dissolution rate [67] [68].
Evolution and Classification of ASDs
ASDs have evolved through four distinct generations:
- First Generation: Utilize crystalline carriers (e.g., urea, sugars). The resulting crystalline solid dispersions are thermodynamically stable but offer a slower dissolution rate compared to amorphous forms [68].
- Second Generation: Employ amorphous polymeric carriers (e.g., PVP, HPMC, PEG). These create metastable supersaturated solutions, leading to faster dissolution, but carry a risk of drug precipitation and recrystallization over time [68].
- Third Generation: Incorporate surfactants or polymers with surface-active properties (e.g., inulin, Gelucire, poloxamer). These excipients help stabilize the supersaturated state and inhibit drug recrystallization, improving both dissolution and physical stability [68].
- Fourth Generation: Controlled-release solid dispersions (CRSDs) that combine solubility enhancement with extended release profiles using water-insoluble or swellable polymers (e.g., ethyl cellulose) [68].
Key Mechanisms of Action
- Energy Enhancement: The amorphous form possesses higher free energy than its crystalline counterpart, lowering the thermodynamic barrier to dissolution [68].
- Wetting and Carrier Dissolution: Hydrophilic carriers improve the wettability of the hydrophobic drug. As the carrier dissolves, the drug is released as fine colloidal particles or in a molecularly dispersed state, creating a supersaturated solution [68].
- Anti-plasticization: The drug can act as an anti-plasticizer, increasing the glass transition temperature (Tg) of the polymer, thereby improving the physical stability of the dispersion by reducing molecular mobility [68].
Manufacturing Techniques and Data
Table 2: Comparison of Major Amorphous Solid Dispersion Manufacturing Techniques
| Manufacturing Technique | Process Brief | Key Process Variables | Advantages | Challenges |
|---|---|---|---|---|
| Hot Melt Extrusion (HME) | API and polymer are mixed, heated, and extruded through a die to form a homogeneous solid dispersion [67]. | Temperature, screw speed/size/design, feed rate [67]. | Continuous process, solvent-free, high throughput [67]. | Requires API stability at high processing temperatures [67]. |
| Spray Drying | API and polymer are dissolved in a common solvent, which is then atomized and rapidly dried to form solid particles [67]. | Solvent choice, inlet/outlet temperature, feed rate, atomization pressure [67]. | Suitable for heat-sensitive drugs, scalable [67]. | Residual solvent removal, high energy consumption [67]. |
| Supercritical Fluid (SCF) Technology | Uses supercritical COâ‚‚ (scCOâ‚‚) as an anti-solvent (e.g., SAS, GAS) or as a solvent (RESS) to precipitate API and polymer [67]. | Pressure, temperature, COâ‚‚ flow rate, nozzle geometry [67]. | Mild temperatures, produce fine particles, minimal solvent residue [67]. | High capital cost, complex process optimization [67]. |
Comparative Pharmacokinetics: Bridging the Gap Between Oral and IV Performance
The ultimate goal of these formulation strategies is to create oral dosage forms whose pharmacokinetic (PK) profiles approach the efficiency of IV administration. IV therapy provides complete bioavailability and immediate systemic exposure but is invasive and costly. Advanced formulations can narrow this PK gap.
Case Study: Busulfan A controlled clinical study compared IV and oral busulfan in patients undergoing hematopoietic stem cell transplantation. While the median AUC over 24 hours during conditioning was similar between the IV (5598.0 µMolmin) and oral (4440 µMolmin) groups, the oral formulation exhibited "extremely variable" pharmacokinetics and clearance. This highlights that even when average exposure is similar, inter-individual variability can be a significant challenge for oral formulations [20].
The Role of Physiologically-Based Pharmacokinetic (PBPK) Modeling PBPK analysis is a powerful tool for evaluating the in vivo relevance of in vitro solubility. For instance, a study on pazopanib demonstrated that using its low in vitro FaSSIF solubility in a PBPK model failed to capture the observed clinical exposure. To match the clinical PK data, a 20-fold increase in the input solubility was required, indicating that the drug's absorption is truly limited by its solubility in vivo. This "totality of evidence" approach helps identify drugs that will benefit most from formulation interventions and can predict food effects [62].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Excipients and Materials for Solubility Enhancement Formulations
| Category | Example Excipients | Function in Formulation |
|---|---|---|
| Lipids for Nanocarriers | Medium-chain triglycerides (MCT), Trimyristin (Dynasan 114), Phosphatidylcholine (Lipoid S 100) [66] | Form the core matrix of lipid nanoparticles and emulsions, solubilizing lipophilic drugs [66]. |
| Surfactants & Stabilizers | Poloxamer 188 (Kolliphor), PEG-lipids (DSPE-PEG) [63] [66] | Stabilize nanoparticle surfaces, prevent aggregation, control particle size, and enhance wettability [63] [66]. |
| Polymers for ASDs | Synthetic: PVP, PVPVA, PEG [68]. Cellulose-based: HPMC, HPMCAS, HPC [67] [68]. Advanced: Soluplus (graft copolymer) [67]. | Act as carriers in ASDs, inhibiting crystallization, maintaining supersaturation, and providing a matrix for molecular dispersion [67] [68]. |
| Surfactants in ASDs | Gelucire, Poloxamer, Inulin [68] | Used in 3rd gen ASDs to inhibit precipitation, stabilize the drug in solution, and enhance dissolution [68]. |
Decision Framework: Selecting a Formulation Strategy
The choice between nanocarriers and ASDs depends on the API's properties, the target product profile, and practical manufacturing considerations. The following diagram outlines a logical decision workflow for selecting a formulation strategy.
Diagram 1: A logical workflow for selecting between nanocarrier and amorphous solid dispersion formulation strategies based on drug properties and goals.
The challenge of poor solubility remains a significant bottleneck in drug development. Both nanocarriers and amorphous solid dispersions offer powerful, validated strategies to overcome this hurdle and enhance the oral bioavailability of BCS Class II and IV drugs. The choice of technology is not one-size-fits-all but should be guided by a systematic evaluation of the API's physicochemical properties, stability, and the desired therapeutic outcome.
Nanocarriers excel for highly lipophilic drugs and when targeted delivery is a priority, leveraging their ability to solubilize drugs in a lipid core or at a lipid-water interface. In contrast, amorphous solid dispersions are particularly effective at creating and maintaining a metastable supersaturated state, leading to rapid dissolution and absorption, with different generations of polymers and surfactants available to mitigate stability risks. The ongoing integration of advanced tools like PBPK modeling and the development of novel excipients will continue to refine these strategies, enabling more predictable and successful development of robust oral formulations that narrow the pharmacokinetic gap with intravenous administration.
The oral route of administration is the most patient-preferred and convenient method for drug delivery, offering advantages in accessibility, compliance, and cost compared to injectable formulations [69]. However, many active pharmaceutical ingredients (APIs) suffer from low oral bioavailability due to two significant biological barriers: pre-systemic first-pass metabolism and active efflux by transport proteins such as P-glycoprotein (P-gp) [70] [16]. First-pass effect refers to the phenomenon where a drug is metabolically inactivated at specific locations—primarily the gut wall and liver—before it reaches the systemic circulation, substantially reducing the active drug concentration [16]. Efflux transporters further limit bioavailability by actively pumping absorbed drugs back into the intestinal lumen [71].
This guide objectively compares two primary strategic approaches—prodrug design and inhibitor co-administration—for circumventing these barriers, framing the analysis within the broader context of comparative pharmacokinetics of oral versus intravenous administration. Intravenous administration provides a critical benchmark for these strategies, as it completely bypasses first-pass metabolism and efflux, resulting in 100% bioavailability and immediate systemic drug availability [72] [73]. The following sections provide a detailed comparison of these technologies, supported by experimental data and methodologies relevant to researchers and drug development professionals.
Strategic Approaches: Mechanisms and Comparisons
Prodrug Technology
Prodrugs are biologically inactive derivatives of active drug molecules designed to overcome pharmaceutical and pharmacokinetic barriers through chemical modification. They undergo enzymatic or chemical transformation in vivo to release the active parent compound [74].
Glyceride-Mimetic Prodrugs for Lymphatic Transport: This innovative approach exploits the intestinal lymphatic system, which drains directly into the systemic circulation via the thoracic duct, thereby bypassing hepatic first-pass metabolism. Glyceride-mimetic prodrugs incorporate self-immolative spacers to conjugate APIs to triglyceride-like structures, facilitating their incorporation into chylomicrons and subsequent transport via the lymphatic pathway [74]. In a proof-of-concept study with testosterone (a high first-pass drug), this strategy resulted in remarkable increases of up to 90-fold in plasma exposure compared to testosterone undecanoate, a commercially available prodrug [74].
Solid Self-Microemulsifying Drug Delivery Systems (S-SMEDDS): These systems combine prodrug principles with advanced formulation to address both solubility and efflux limitations. S-SMEDDS are isotropic mixtures of oil, surfactant, and co-surfactant that form fine oil-in-water microemulsions upon aqueous dilution in the gut. The formulation can enhance absorption by maintaining drug solubility and inhibiting intestinal transporters like P-gp [71]. For relugolix, an S-SMEDDS formulation demonstrated a 1.9-fold increase in oral bioavailability compared to conventional suspensions in pharmacokinetic studies [71].
Inhibitor Co-Administration
This strategy involves the co-administration of an API with another compound that specifically inhibits metabolic enzymes or efflux transporters.
P-gp Inhibition via Formulation Excipients: Some excipients used in advanced delivery systems possess inherent P-gp inhibitory activity. In the relugolix S-SMEDDS study, the formulation itself was shown to enhance drug absorption partly through the inhibition of intestinal transporters, reducing efflux back into the gut lumen [71]. This demonstrates how inhibitor co-administration can be seamlessly integrated into the formulation strategy rather than requiring a separate therapeutic agent.
Competitive Enzyme Inhibition: The oral bioavailability of drugs vulnerable to cytochrome P450 (CYP) metabolism, particularly CYP3A4, can be increased if another drug competing for the same metabolic enzymes is given concurrently. For example, the first-pass extraction of propranolol can be reduced when administered with chlorpromazine [16]. This pharmacokinetically driven approach, however, raises considerations of potential drug-drug interactions.
Quantitative Comparison of Strategic Outcomes
Table 1: Comparative Bioavailability Enhancement of Different Strategies
| Strategy | Model Drug | Experimental System | Bioavailability Outcome | Key Mechanism |
|---|---|---|---|---|
| Glyceride-Mimetic Prodrug | Testosterone | Rat model | Up to 90-fold increase vs. testosterone undecanoate [74] | Lymphatic transport bypassing hepatic first-pass [74] |
| S-SMEDDS | Relugolix | In vivo pharmacokinetic study | 1.9-fold increase vs. drug suspension [71] | P-gp inhibition & improved solubility [71] |
| IV Administration (Benchmark) | Topotecan | Rat model (4 mg/kg dose) | 100% bioavailability (by definition) [75] | Complete direct entry into systemic circulation [75] |
| Subcutaneous Administration | Topotecan | Rat model (4 mg/kg dose) | 88.05% (lactone) to 99.75% (total) [75] | Sustained release, partial avoidance of first-pass [75] |
Visualizing the Key Pathways and Strategies
The following diagram illustrates the biological pathways of drug absorption and the strategic points of intervention for prodrugs and inhibitors.
Diagram 1: Drug absorption pathways and strategic interventions for overcoming first-pass metabolism and efflux. Strategies (blue) target specific barriers (red) to enhance systemic drug delivery.
Experimental Data and Pharmacokinetic Comparisons
Intravenous Administration as the Pharmacokinetic Benchmark
Intravenous (IV) administration provides the gold standard for 100% bioavailability as it introduces drugs directly into the systemic circulation, completely avoiding absorption barriers and first-pass metabolism [72]. This makes IV dosing crucial for determining absolute oral bioavailability and provides a benchmark against which oral strategies are measured.
Table 2: Route-Dependent Pharmacokinetic Parameters of Topotecan (4 mg/kg dose in rats) [75]
| Parameter | Lactone Form (Active) | Total Topotecan |
|---|---|---|
| Bioavailability | ||
| Â Â Â Intravenous (IV) | 100% (by definition) | 100% (by definition) |
| Â Â Â Subcutaneous (SC) | 88.05% | 99.75% |
| Â Â Â Oral (PO) | Significantly lower than SC | Significantly lower than SC |
| Plasma Concentration at Elimination Phase | ||
| Â Â Â Subcutaneous (SC) vs. Oral (PO) | ~2x higher | ~2x higher |
| Â Â Â Subcutaneous (SC) vs. Intravenous (IV) | ~10x higher | ~10x higher |
The data in Table 2 demonstrates that subcutaneous administration of topotecan provides a sustained-release profile, resulting in higher plasma concentrations during the elimination phase compared to both oral and IV routes [75]. This highlights how alternative administration routes can significantly alter pharmacokinetic profiles beyond simply improving bioavailability.
Bioavailability Comparisons Across Drug Classes
Table 3: Absolute Oral Bioavailability of Various Drugs [31] [3] [16]
| Drug | Absolute Oral Bioavailability | Primary Reason for Low/Reduced Bioavailability |
|---|---|---|
| Ibuprofen | 91% [31] [3] | Low first-pass loss |
| Tramadol | 80% [31] [3] | First-pass effect (~30%) [3] |
| Nitroglycerin | Very low (<1%) [16] | High hepatic first-pass metabolism [16] |
| Benzylpenicillin | Low | Degradation in gut wall [16] |
| Insulin | Negligible | Gut wall metabolism and molecular size [16] |
| Remdesivir | Negligible | Extensive trapping and metabolism in liver [16] |
Drugs like ibuprofen with high native oral bioavailability have less need for advanced circumvention strategies. In contrast, drugs like nitroglycerin, insulin, and remdesivir, which face extreme first-pass metabolism or degradation, are prime candidates for prodrug design, inhibitor co-administration, or development for non-oral routes [16].
Detailed Experimental Protocols
Protocol: In Vivo Pharmacokinetic Study for Bioavailability Assessment
This protocol is adapted from methodologies used to compare administration routes for drugs like topotecan and Ibuprofen/Tramadol combinations [75] [31] [3].
Objective: To determine and compare the absolute bioavailability and pharmacokinetic parameters of a drug candidate after oral, intravenous, and subcutaneous administration.
Methodology:
- Study Design: Randomized, open-label, crossover design where each subject/animal receives all treatments with a sufficient washout period between doses to avoid carryover effects [31] [3].
- Formulations:
- IV Solution: For 100% bioavailability reference. The drug is dissolved in a suitable solvent (e.g., saline) and filtered through a sterile filter [75].
- Oral Formulation: Test prodrug or inhibitor combination (e.g., S-SMEDDS, glyceride-mimetic) vs. control (e.g., drug suspension) [75] [71].
- SC Formulation: Aqueous solution or suspension of the drug [75].
- Dosing and Sampling: Animals (e.g., rats, n=5 per group) receive a single dose (e.g., 4 mg/kg for topotecan). Serial blood samples are collected at pre-determined time points (e.g., pre-dose, 0.25, 0.5, 1, 2, 4, 6, 8, 12, 24 hours post-dose) [75].
- Sample Analysis: Plasma is separated and analyzed for drug concentrations (both lactone and total forms if applicable) using a validated bioanalytical method, such as UPLC-ESI-MS/MS [75].
- Pharmacokinetic Analysis: Non-compartmental analysis is performed to calculate key parameters:
- AUC
0-t, AUC0-∞: Area under the plasma concentration-time curve. - C
max: Maximum plasma concentration. - T
max: Time to reach Cmax. - t
1/2: Elimination half-life. - F (Absolute Bioavailability): Calculated as (AUC
oral* DoseIV) / (AUCIV* Doseoral) * 100% [75].
- AUC
Protocol: In Vitro Cellular Uptake and Transport Studies
This protocol is used to evaluate the mechanism of absorption enhancement, particularly for P-gp inhibition, as seen in relugolix S-SMEDDS research [71].
Objective: To assess the cellular uptake and permeability of a drug and investigate the role of efflux transporters.
Methodology:
- Cell Culture: Use Caco-2 cell lines (human colon adenocarcinoma), cultured and seeded on semi-permeable Transwell inserts. Allow 21-25 days for full differentiation into a polarized monolayer mimicking the intestinal epithelium [71].
- Transepithelial Electrical Resistance (TEER): Measure TEER before and after experiments to confirm monolayer integrity.
- Transport Experiment:
- Apply the test formulation (e.g., S-SMEDDS, drug with/without inhibitor) to the donor compartment (apical for A→B transport, or basolateral for B→A transport).
- Sample from the receiver compartment at regular intervals over 2-3 hours.
- Analyze samples via HPLC or MS to determine drug concentration.
- Data Analysis: Calculate the apparent permeability (P
app) and the efflux ratio (Papp,B→A/ Papp,A→B). A reduction in the efflux ratio in the presence of the test formulation indicates inhibition of active efflux transporters [71].
Visualizing the Experimental Workflow
The following diagram outlines the key steps in evaluating a novel formulation's ability to enhance oral bioavailability.
Diagram 2: Experimental workflow for evaluating bioavailability enhancement strategies, integrating in vitro and in vivo pharmacokinetic studies.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 4: Key Reagents and Materials for Bioavailability Enhancement Studies
| Reagent / Material | Function / Application | Example Use Case |
|---|---|---|
| Caco-2 Cell Line | An in vitro model of the human intestinal mucosa for predicting drug absorption and studying efflux transport [71]. | Used in transwell assays to determine apparent permeability (Papp) and efflux ratio for relugolix formulations [71]. |
| UPLC-ESI-MS/MS System | A highly sensitive and specific bioanalytical instrument for quantifying drug concentrations in biological matrices (plasma, urine, feces) [75]. | Used to quantify lactone and total forms of topotecan in rat plasma for pharmacokinetic analysis [75]. |
| Solutol HS 15 | A non-ionic surfactant used in self-microemulsifying drug delivery systems (SMEDDS) to enhance solubility and inhibit P-gp [71]. | A key component (49% w/w) of the optimized relugolix S-SMEDDS formulation [71]. |
| Transcutol HP | A high-purity diethylene glycol monoethyl ether used as a co-surfactant/cosolvent in SMEDDS to improve drug solubilization [71]. | A component (25% w/w) of the relugolix S-SMEDDS formulation [71]. |
| Repaglinide | A compound used as an internal standard (IS) in mass spectrometry to normalize and improve the accuracy of quantitative analysis [75]. | Served as the IS for the UPLC-ESI-MS/MS bioanalysis of topotecan in biological samples [75]. |
| Design-Expert Software | A statistical software package used for formulation optimization via designs like Response Surface Methodology (RSM) [71]. | Used to optimize the composition of the relugolix SMEDDS based on factors like solubility and emulsification efficiency [71]. |
The strategic circumvention of first-pass metabolism and efflux is a cornerstone of modern drug development, essential for unlocking the therapeutic potential of countless APIs with poor inherent oral bioavailability. As the data and protocols presented herein demonstrate, prodrug design and inhibitor co-administration offer powerful, yet distinct, pathways to this goal.
- Prodrug strategies, particularly those enabling lymphatic transport or integrated into advanced delivery systems like S-SMEDDS, can achieve dramatic improvements in systemic exposure—up to 90-fold in the case of glyceride-mimetic testosterone [74].
- Inhibitor co-administration, whether via specific therapeutic agents or functional excipients, provides a more direct mechanism to block the efflux and metabolic enzymes responsible for drug loss [71] [16].
The choice between these strategies, or their potential combination, must be guided by the specific physicochemical and pharmacokinetic properties of the drug candidate. Intravenous administration remains the indispensable benchmark for these evaluations, providing the critical 100% bioavailability reference for calculating absolute oral bioavailability and understanding the true impact of these advanced technologies [75] [72]. As the field progresses, the integration of more predictive human-relevant models and sophisticated drug delivery systems will continue to refine these approaches, ultimately expanding the realm of drugs that can be effectively delivered via the preferred oral route.
The pursuit of optimal pharmacokinetic (PK) profiles represents a central challenge in modern drug development. Controlled-release (CR) technologies, particularly matrix tablets and delayed-release coatings, are critical tools for achieving this goal, enabling precise temporal and spatial control over drug delivery. This comparison guide provides a systematic evaluation of these technologies, framing their performance within a broader thesis on comparative pharmacokinetics of oral versus intravenous administration. For researchers and drug development professionals, this analysis synthesizes current experimental data, detailed methodologies, and key reagent solutions to inform rational formulation design, highlighting how advanced oral systems can bridge the performance gap with parenteral routes by improving bioavailability, reducing dosing frequency, and mitigating side effects.
The fundamental objective of any drug delivery system is to achieve and maintain a therapeutic drug concentration at the site of action for a desired duration. While intravenous (IV) administration provides complete bioavailability and immediate systemic exposure, its clinical utility is often limited by invasiveness, patient discomfort, and the risk of concentration-dependent toxicity. Oral administration remains the most patient-preferred and convenient route; however, it must overcome significant physiological barriers, including first-pass metabolism, variable gastrointestinal (GI) transit, and pH-dependent stability [3] [76].
Controlled-release technologies are engineered to optimize the pharmacokinetic (PK) profiles of orally administered drugs, making them more predictable and therapeutically advantageous. Using a combination of analgesics allows for the use of lower doses of each, therefore, lowering the risk of side effects, a principle that can be extended to optimizing release profiles from a single drug [3]. These systems provide drug release in an amount sufficient to maintain therapeutic levels over an extended period, with the release profiles predominantly controlled by the system's design rather than external environments [77]. This review focuses on two cornerstone CR technologies: matrix tablets, where the drug is dispersed within a polymer network that controls its release, and delayed-release coatings, which utilize a polymer barrier to target release to specific regions of the GI tract. By examining their mechanisms, performance data, and experimental protocols, this guide aims to equip scientists with the knowledge to select and optimize the appropriate technology for specific drug candidates.
The release behavior of CR systems is inherently non-linear, with continuously diminishing release rate due to diffusional resistance and/or a decrease in effective area at the diffusion front [77]. Understanding the distinct mechanisms of matrix and coated systems is essential for predicting their in vivo performance.
Matrix Tablet Systems
Matrix systems are among the most popular and cost-effective means for oral controlled drug delivery [76]. In a hydrophilic matrix system, the release mechanism initiates upon water penetration into the matrix, triggering polymer hydration and swelling, forming a gel layer. Drug release then occurs via dissolution, diffusion through the gel layer, and eventual erosion of the gel [76]. These systems are characterized by multiple 'fronts': a diffusion front between the swelling and erosion fronts, which governs the release kinetics [76]. The kinetics can be described by the Power Law equation: M_t / M_∞ = k * t^n, where n is the diffusional exponent indicative of the release mechanism (Fickian diffusion if n=0.5, Case-II transport or zero-order if n=1) [77].
Delayed-Release Coating Systems
Delayed-release coatings, such as enteric coatings, act as a barrier that remains intact in the acidic environment of the stomach but dissolves in the higher pH of the intestine (e.g., Eudragit S100 dissolves at pH >7) [78]. The primary mechanism is the dissolution of the polymer film at a specific pH threshold, leading to a pulsed release after a defined lag time. The integrity and solubility of this polymeric film are critical for performance, governed by the polymer's physicochemical properties, including tensile strength, contact angle (wettability), and solubility in different media [78].
The diagram below illustrates the core mechanisms and experimental workflow for evaluating these two technologies.
Performance Data and Experimental Comparison
Quantitative Comparison of Polymer Performance
The selection of the polymer is arguably the most critical factor in determining the release profile. The following table summarizes experimental data on the key physical properties and release-controlling efficiency of common polymers used in these technologies.
Table 1: Physical Properties and Release-Controlling Efficiency of Common Polymers [78]
| Polymer | Tensile Strength (MPa) | Contact Angle (°) | Weight Loss in SIF (%) | Drug Release Rate (mg/h¹/²) | Primary Release Mechanism | Common Application |
|---|---|---|---|---|---|---|
| Ethylcellulose (EC) | Highest | Highest | Lowest | 6.45 (Fastest) | Diffusion-controlled, sustained | Hydrophobic Matrix |
| Eudragit RS100 | Medium | Medium | Medium | 5.23 | Diffusion-controlled, sustained | Permeable Coating |
| HPMC 100000 | Medium | Low | Low | 4.10 | Swelling & Diffusion | Hydrophilic Matrix |
| HPMC 4000 | Low | Lowest | High | 3.45 | Swelling & Erosion | Hydrophilic Matrix |
| Eudragit S100 | High | High | pH-Dependent | 1.11 (Slowest, pH-dependent) | Pulsed (Enteric) | Delayed-Release Coating |
Pharmacokinetic Outcomes: Oral CR vs. Intravenous Administration
The ultimate test of a CR technology is its ability to produce a favorable and predictable PK profile in vivo. A recent randomized clinical trial directly comparing IV and oral administration of a combined ibuprofen/tramadol formulation provides a clear illustration of the PK bridge that CR technologies aim to construct.
Table 2: Pharmacokinetic Parameters from IV vs. Oral Administration of Ibuprofen/Tramadol [3]
| Analyte / Parameter | Administration Route | Cmax | Tmax (h) | AUC (ng·h/mL) | Absolute Bioavailability | Key Findings |
|---|---|---|---|---|---|---|
| Ibuprofen | Intravenous (IV) | Higher | Immediate | Equivalent | 91% | Equivalent AUC indicates complete absorption. |
| Oral | Lower | Slower | Equivalent | |||
| Tramadol | Intravenous (IV) | Higher | Immediate | Higher | 80% | IV produces higher parent drug exposure. |
| Oral | Lower | Slower | Lower | |||
| M1 Metabolite | Intravenous (IV) | Lower | Slower | Lower | N/A | Oral route generates higher active metabolite exposure. |
The data from this study highlights several critical points for formulation scientists. First, ibuprofen's high oral bioavailability (91%) and equivalent AUC between routes make it an excellent candidate for oral CR formulation, as little drug is lost to absorption limitations. Second, for Tramadol, the lower oral bioavailability and different metabolite profile mean that an oral CR product is not a direct 1:1 substitute for IV dosing; the formulation must be designed with these PK differences in mind. The study concluded that upon switching from oral to IV dosing, the Tramadol dose should not be reduced, a crucial insight for clinical bridging studies [3].
Detailed Experimental Protocols
To ensure reproducibility and robust data generation, the following section outlines standardized experimental protocols for evaluating controlled-release formulations.
Protocol for Formulation and Characterization of Matrix Tablets
This protocol is adapted from research on hydrophilic and hydrophobic matrix systems [77] [78].
- Matrix Tablet Preparation (Direct Compression): Accurately weigh the drug substance (e.g., Acetaminophen as a model drug), polymer (e.g., HPMC K4MP, EC), and excipients (e.g., diluent, disintegrant). Blend the powders in a twin-shell blender for 15-30 minutes. Add the lubricant (e.g., magnesium stearate) and blend for an additional 5 minutes. Compress the final blend into tablets using a suitable tablet press with flat-faced round punches. Key formulation variables include drug-to-polymer ratio (D:P) and polymer viscosity grade.
- Physical Characterization:
- Tensile Strength: Test preconditioned tablets or casted films using a Universal Testing Machine with an extension speed of 1.0 mm/min, based on the ASTM D638 method. Calculate tensile strength from the recorded stress-strain curve [78].
- Surface Morphology: Analyze the surface and cross-section of tablets or films using Scanning Electron Microscopy (SEM) after sputter-coating with gold to evaluate microstructure and integrity.
- In Vitro Drug Release Testing: Use USP Dissolution Apparatus I (baskets) or II (paddles). A common protocol involves 900 mL of dissolution medium maintained at 37 ± 0.5°C with a paddle speed of 50-100 rpm. For bio-relevant testing, use a sequential pH-change method: simulated gastric fluid (SGF) without enzymes for 2 hours, followed by simulated intestinal fluid (SIF) without enzymes for the remainder of the test. Withdraw samples at predetermined time intervals and analyze drug concentration using HPLC or UV-Vis spectrophotometry [78].
- Release Kinetics Modeling: Fit the obtained release data to various mathematical models to identify the predominant release mechanism:
- Zero-Order:
W = k_0 * t - Higuchi (Square Root):
W = k_H * t^(1/2) - Korsmeyer-Peppas (Power Law):
M_t / M_∞ = k * t^n[77]
- Zero-Order:
Protocol for Application and Evaluation of Delayed-Release Coatings
This protocol is based on studies evaluating polymeric film coatings [78].
- Coating Solution Preparation: Dissolve the polymer (e.g., Eudragit S100, Eudragit RS100) in appropriate organic solvents (e.g., acetone, isopropanol) to achieve a 5-10% w/w solid content. Add a plasticizer (e.g., Dibutyl Sebacate, 20% based on polymer weight) and anti-tacking agents (e.g., talc). Stir the solution for 24 hours to ensure complete plasticization and homogeneity.
- Coating Process (Pan Coating): Load core tablets into a perforated coating pan. Pre-heat the tablets to approximately 30-40°C. Apply the coating solution using a spray gun under controlled conditions: spray rate (e.g., 2-5 mL/min), atomizing air pressure (e.g., 1-2 bar), and inlet air temperature (e.g., 40-50°C). Continue the process until the desired coating weight gain (e.g., 2-10%) is achieved.
- Coating Film Characterization:
- Contact Angle: Prepare a spin-coated thin film of the polymer on a silicon wafer. Measure the contact angle of a deionized water droplet using a goniometer by the sessile drop technique to determine film wettability [78].
- Weight Loss (Film Solubility): Accurately weigh (W1) a casted film. Immerse it in 900 mL of release medium (SGF or SIF) at 37°C in a dissolution apparatus (basket, 100 rpm) for 3 hours. Remove the film residue, dry it to a constant mass (W2), and calculate weight loss as
(W1 - W2)/W1 * 100[78].
- In Vitro Drug Release Testing: Perform dissolution testing as described in Section 4.1, specifically using the sequential pH-change method to verify the delayed release (i.e., no release in SGF for 2 hours, followed by rapid and complete release in SIF).
The Scientist's Toolkit: Essential Research Reagents and Materials
The following table catalogs key materials and their functions essential for R&D in controlled-release technologies.
Table 3: Essential Research Reagents and Materials for CR Formulation Development
| Material / Reagent | Function / Role | Specific Examples |
|---|---|---|
| Polymer - HPMC | Hydrophilic matrix former; swells in water to form a gel layer controlling drug release. | Methocel K4MP (HPMC 4000), Methocel K100MP (HPMC 100000) [78] |
| Polymer - Ethylcellulose | Hydrophobic matrix former / coating; insoluble polymer that controls release via diffusion through pores. | Ethocel Standard Premium, Ethocel Standard FP Premium [77] |
| Polymer - Polymethacrylates | Cationic/Anionic copolymers for sustained or delayed (enteric) release coatings. | Eudragit RS100 (sustained), Eudragit S100 (enteric) [78] |
| Plasticizer | Increases flexibility and durability of polymer films by reducing glass transition temperature. | Dibutyl Sebacate (DBS), Triethyl Citrate [78] |
| Anti-Tacking Agent | Prevents agglomeration of coated tablets during the coating process. | Talc (purified magnesium silicate) [78] |
| Dissolution Media | Simulate gastrointestinal fluids for in vitro release testing. | Simulated Gastric Fluid (SGF, pH 1.2), Simulated Intestinal Fluid (SIF, pH 6.8) [78] |
| Model Drug Substances | Biopharmaceutics Classification System (BCS) model drugs for release studies. | Acetaminophen (APAP, BCS I/III), Ibuprofen (BCS II) [3] [78] |
Integrated Discussion and Formulation Strategy
The choice between a matrix system and a coated system is not merely a technical preference but a strategic decision driven by the drug's physicochemical properties and the target pharmacokinetic profile. The experimental data demonstrates that Ethylcellulose and Eudragit RS100 are highly effective for producing sustained, diffusion-controlled release, while HPMC-based matrices offer a robust and cost-effective platform for swelling-controlled release [78]. For site-specific delivery, such as protecting an acid-labile drug or targeting the intestine, enteric polymers like Eudragit S100 are indispensable.
The comparative PK data of oral versus IV administration underscores a critical principle: a well-designed oral controlled-release system can achieve systemic exposure (AUC) equivalent to an IV formulation, as seen with Ibuprofen [3]. The primary difference lies in the rate of absorption, reflected in a lower Cmax and delayed Tmax, which is often therapeutically desirable as it minimizes peak-related side effects and provides a smoother plasma concentration-time profile.
When designing a CR formulation, scientists must adopt an integrated approach. The workflow should begin with a thorough analysis of the drug's properties (solubility, pKa, half-life). This informs the selection of the appropriate technology (matrix vs. coating) and polymer. The formulation is then optimized using a Design of Experiments (DoE) approach, and the in vitro release profile is rigorously characterized. Finally, the in vivo performance should be validated through pharmacokinetic studies, always considering the context of the drug's clinical pharmacology and the intended therapeutic outcome.
For drug development professionals, the oral route remains the most favorable and preferred for drug administration, with over 60% of marketed drugs being oral products [79]. However, this path is fraught with challenges, as the journey of an orally administered drug through the gastrointestinal (GI) tract is "perilous" and its bioavailability is modulated by numerous factors [80]. The fundamental principle of oral pharmacokinetics (PK) hinges on the drug's absorption—the transportation of the unmetabolized drug from the administration site to the body circulation system [8]. Unlike intravenous administration which achieves 100% bioavailability by directly entering systemic circulation, oral drugs must survive encounters with low pH, GI secretions, degrading enzymes, and cross multiple biological barriers before reaching systemic circulation [8] [11].
The therapeutic effectiveness and safety of a drug are directly determined by its concentration in plasma, which in turn depends on the rate and extent of drug absorption [8]. Understanding and mitigating the variability in this process is therefore critical in drug development. This review examines the primary sources of variability in oral drug pharmacokinetics and contrasts them with intravenous administration, providing researchers with evidence-based strategies to optimize drug delivery systems and clinical outcomes.
Physiological Variability in the Gastrointestinal Tract
The gastrointestinal tract presents a highly dynamic environment with significant interindividual variability that directly impacts drug absorption. The GI tract can be conceptualized as a muscular tube extending from mouth to anus, but in reality, it represents a complex absorption environment with regional differences that profoundly influence drug bioavailability [80].
Regional Variability and Transit Times
Drug absorption along the GI tract is not uniform. The small intestine serves as the primary site for absorption of most drugs due to its extensive surface area, recently estimated at approximately 32 m² [80]. This large surface area, created by villi and microvilli, facilitates enhanced drug absorption compared to other GI regions. However, significant interindividual variability exists in GI dimensions, with small intestine length ranging from 298-1030 cm and colon length varying from 80-313 cm between individuals [80].
Transit times through different GI segments also show remarkable variability:
- Gastric emptying time: Highly variable and influenced by food, especially fatty food which slows emptying [11]
- Small intestinal transit: Typically ranges from 2-6 hours [80]
- Colonic transit: Can vary dramatically from 6-70 hours between individuals [80]
These transit times critically influence absorption, particularly for drugs that are absorbed by active transport, dissolve slowly, or are polar with low lipid solubility [11]. The rate of gastric emptying often becomes the rate-limiting step in drug absorption, as most absorption occurs in the small intestine [11].
pH Variability and Membrane Permeability
The pH environment varies considerably along the GI tract, from highly acidic in the stomach (pH ~1.4) to progressively more alkaline in the small intestine, approaching pH 8 in the lower ileum [11]. This pH gradient significantly impacts drug absorption, as most drugs are weak organic acids or bases that exist in both ionized and un-ionized forms [11].
The un-ionized form typically exhibits higher lipid solubility and can diffuse readily across cell membranes, while the ionized form has low lipid solubility and high electrical resistance, limiting membrane penetration [11]. The proportion of un-ionized to ionized drug is determined by environmental pH and the drug's pKa (acid dissociation constant). This relationship explains why weakly acidic drugs are more readily absorbed from the stomach, while weakly basic drugs are predominantly absorbed in the higher pH environment of the small intestine [11].
Table 1: Key Physiological Factors Contributing to Oral Drug PK Variability
| Physiological Factor | Interindividual Variability | Impact on Drug Absorption |
|---|---|---|
| GI Tract Length | Small intestine: 298-1030 cm; Colon: 80-313 cm [80] | Determines absorptive surface area and residence time |
| Transit Times | Gastric: highly variable; Small intestine: 2-6 h; Colon: 6-70 h [80] | Affects duration for drug dissolution and absorption |
| Luminal pH | Stomach: ~1.4; Duodenum: 4-5; Ileum: ~8 [11] | Influences drug ionization and membrane permeability |
| Mucus Layer | Varies by region: stomach (double layer), small intestine (single layer), colon (double layer) [80] | Creates diffusion barrier for drug molecules |
| Blood Flow | Reduced in shock/critically ill patients [8] | Lowers concentration gradient across intestinal mucosa |
Mechanisms of Drug Absorption and Transport
Understanding the fundamental mechanisms by which drugs cross biological membranes is crucial for predicting and mitigating variability in oral pharmacokinetics.
Primary Transport Mechanisms
The passage of drugs across intestinal membranes occurs through several distinct mechanisms:
Passive Diffusion: This is the most common mechanism where drug molecules move according to concentration gradient from higher to lower concentration until equilibrium is reached [8]. Lipid diffusion is particularly important due to the lipid barriers separating body compartments [8].
Carrier-Mediated Membrane Transport: Specialized transport systems exist for ions and nutrients, including:
Receptor-Mediated Endocytosis: Internalization process where extracellular molecules bind to receptors and are transported across cells [79]. This mechanism is particularly relevant for macromolecular drugs.
Pinocytosis: Process where fluid or particles are engulfed by cells through vesicle formation, requiring energy expenditure [11].
Transporter Systems and Their Implications
Various transporter families play significant roles in drug absorption:
Table 2: Key Drug Transporter Families and Their Functions
| Transporter Family | Examples | Function | Substrate Examples |
|---|---|---|---|
| Organic Cation Transporters (OCTs) | OCT1, OCT2, OCT3 [81] | Uptake of small, positively charged compounds | Metformin, platinum antineoplastics [81] |
| Organic Anion Transporters (OATs) | OAT1, OAT3 [81] | Move small organic anions using Na+ gradient | Antibiotics, antivirals, angiotensin-converting enzyme inhibitors [81] |
| Peptide Transporters (PEPTs) | PEPT1 [81] | Proton-coupled di/tri-peptide transport | β-lactam antibiotics, valacyclovir [81] |
| Organic Anion Transporting Polypeptides (OATPs) | OATP1B1, OATP1B3 [81] | Transport amphipathic molecules | Statins, rifampicin, methotrexate [81] |
| ATP-binding Cassette (ABC) | P-glycoprotein (P-gp) [8] | Energy-dependent efflux transport | Various drugs including chemotherapeutic agents [8] |
These transporter systems contribute significantly to absorption variability, as they can be inhibited or induced by concomitant medications, herbs, or food components, leading to potential drug interactions [81].
Quantitative Comparison: Oral versus Intravenous Administration
Comparative pharmacokinetic studies provide critical insights into the differences between oral and intravenous administration, highlighting the unique challenges associated with each route.
Bioavailability and Absorption Kinetics
The fundamental distinction between oral and intravenous administration lies in bioavailability—the fraction of administered drug that reaches systemic circulation intact [8]. While intravenous administration achieves 100% bioavailability by direct entry into systemic circulation, oral drugs must first navigate the GI environment and hepatic first-pass metabolism [8].
A comparative study of chloramphenicol in infants with Haemophilus influenzae meningitis demonstrated key pharmacokinetic differences between routes [19]. Following intravenous administration, the mean peak serum level of 15.0 μg/mL was reached at 45 minutes, while the same dose administered orally achieved a higher mean peak serum level of 18.5 μg/mL but took 2-3 hours to reach this maximum [19]. The mean serum half-life was significantly longer after oral administration (6.5 hours) compared to intravenous (4.0 hours), occasionally leading to drug accumulation with oral dosing [19].
Table 3: Pharmacokinetic Comparison of Oral vs. Intravenous Chloramphenicol in Pediatric Meningitis [19]
| Parameter | Intravenous Administration | Oral Administration | Statistical Significance |
|---|---|---|---|
| Peak Serum Level | 15.0 μg/mL | 18.5 μg/mL | Not specified |
| Time to Peak | 45 minutes | 2-3 hours | Not specified |
| Serum Half-life | 4.0 hours | 6.5 hours | P < 0.001 |
| CSF Trough Levels | 4.2 μg/mL | 6.6 μg/mL | P < 0.001 |
Clinical Implications of Administration Route
The choice between oral and intravenous administration involves careful consideration of clinical circumstances:
Intravenous therapy is recommended for:
- Severe life-threatening infections [82]
- Deep-seated infections with concerns about achieving adequate antibiotic concentrations [82]
- Patients unable to absorb or take oral drugs [82]
- Immunocompromised patients [82]
Oral therapy is preferred for:
- Convenience and patient compliance [82] [80]
- Avoiding cannula-related infections or thrombophlebitis [82]
- Reducing drug administration costs [82]
- Enabling early discharge from hospital [82]
Recent research challenges traditional paradigms, suggesting that earlier switching from intravenous to oral therapy may be feasible for various infections including pneumonia, complicated urinary tract infections, intra-abdominal infections, Gram-negative bacteraemia, and skin and soft tissue infections [82]. The Oral versus Intravenous Antibiotics for Bone and Joint Infection (OVIVA) trial even demonstrated that early switching (within one week) from intravenous to oral therapy was non-inferior to continuing intravenous antibiotics for at least six weeks in bone and joint infections [82].
Key Experimental Protocols for Assessing Oral PK Variability
For researchers investigating oral pharmacokinetic variability, several established experimental approaches provide robust methodology for characterizing absorption patterns.
Protocol: Comparative Oral/IV Bioavailability Study
Objective: To determine absolute bioavailability and characterize absorption profile of an oral formulation relative to intravenous administration.
Methodology:
- Study Design: Randomized, crossover design with adequate washout period [19]
- Participants: 14 infants with Haemophilus influenzae meningitis (as exemplar) [19]
- Dosing Regimen:
- Sample Collection: Multiple serum levels after IV dose (day 4) and after oral dose (day 10) [19]
- CSF Sampling: Measure trough levels six hours after IV or oral dose [19]
- Analysis: Determine peak serum levels, time to peak, elimination half-life, and area under curve (AUC)
Key Parameters:
- Absolute bioavailability (F) = AUCoral / AUCIV × (DoseIV / Doseoral) [8]
- Peak concentration (Cmax) and time to peak (Tmax)
- Elimination half-life (t_½) for each route [19]
Protocol: GI Transit and Absorption Site Characterization
Objective: To evaluate regional drug absorption patterns along the gastrointestinal tract.
Methodology:
- Formulation Approach: Utilize specialized delivery systems (hydrogels, nanoparticles) designed for site-specific release [80]
- Transit Monitoring: Use techniques such as gamma scintigraphy to monitor formulation transit [83]
- Regional Sampling: Employ intestinal aspiration tubes or remote capsules for localized sampling [83]
- pH Monitoring: Concurrent measurement of luminal pH at different GI segments [11] [83]
- Analysis: Correlate regional drug concentrations with absorption metrics
Protocol: Transporter-Mediated Absorption Assessment
Objective: To characterize the role of specific transport systems in drug absorption.
Methodology:
- In Vitro Models: Use transfected cell systems (e.g., Caco-2, MDCK) overexpressing specific transporters [81]
- Inhibition Studies: Assess absorption with and without specific transporter inhibitors [81]
- Genotyping: Screen for genetic polymorphisms in key transporter genes [80] [81]
- Correlation Analysis: Relate transporter expression/function to absorption parameters [81]
Visualization of Oral Drug Absorption Pathways
The following diagram illustrates the key processes and variability factors in oral drug absorption, highlighting the complex journey from administration to systemic circulation:
Diagram 1: Oral Drug Absorption Pathways and Variability Factors
This visualization highlights the complex interplay between drug properties, physiological processes, and patient-specific factors that collectively determine oral drug absorption efficiency and variability. Key modifiable factors (genetics, transporter systems) are highlighted to emphasize potential targets for variability mitigation.
The Scientist's Toolkit: Key Research Reagents and Solutions
For researchers investigating oral pharmacokinetic variability, several essential tools and reagents facilitate comprehensive absorption studies.
Table 4: Essential Research Reagents for Oral Absorption Studies
| Research Tool/Reagent | Function/Application | Key Considerations |
|---|---|---|
| Caco-2 Cell Lines | In vitro model of human intestinal absorption | Express various transporters; correlation with human absorption [79] |
| Transfected Cell Systems | Study specific transporter functions (OATP, OAT, OCT, P-gp) | Overexpress single transporter for mechanistic studies [81] |
| Parallel Artificial Membrane Permeability Assay (PAMPA) | High-throughput passive permeability screening | Predicts transcellular passive diffusion [8] |
| Specific Transporter Inhibitors | Elucidate transport mechanisms (e.g., rifampicin for OATPs) | Confirm involvement of specific transporters [81] |
| Remote Control Capsules | Site-specific drug delivery and sampling in human GI tract | Allows regional absorption assessment [83] |
| Gamma Scintigraphy Imaging | Visualize and quantify GI transit of formulated products | Correlates location with absorption [83] |
| Physiologically-Based PK (PBPK) Modeling Software | Simulate and predict absorption incorporating physiological variability | Integrates multiple variability factors [84] |
| Genotyping Assays | Identify genetic polymorphisms in transporters and metabolizing enzymes | Explains interindividual variability [80] |
The journey of an orally administered drug through the gastrointestinal tract is influenced by a complex interplay of physiological factors, patient characteristics, and drug properties. This review has highlighted the significant variability inherent in oral drug absorption and contrasted it with the more predictable pharmacokinetics of intravenous administration.
For drug development professionals, several strategic approaches can help mitigate this variability:
- Formulation Strategies: Utilization of controlled-release forms, enzyme inhibitors, and absorption enhancers can help overcome biological barriers [79]
- Patient Stratification: Implementation of pharmacogenetic testing for known variants in transporters and metabolizing enzymes can identify subpopulations with different absorption profiles [80]
- Food Effect Management: Systematic evaluation of food effects on absorption to provide optimized dosing recommendations [11]
- IV-to-Oral Switching Protocols: Development of evidence-based guidelines for early transition from intravenous to oral therapy where appropriate [82]
As drug development advances, the integration of physiologically-based pharmacokinetic modeling, advanced in vitro systems, and personalized medicine approaches will continue to enhance our ability to predict and control oral drug absorption variability, ultimately leading to more effective and reliable therapeutic outcomes.
The shift from intravenous (IV) to oral administration of anticancer drugs represents a significant paradigm change in cancer treatment, offering patients greater convenience, improved quality of life, and reduced healthcare system burdens [50] [85]. However, this transition faces substantial pharmacological hurdles, primarily centered on achieving adequate and consistent oral bioavailability [86]. Oral bioavailability refers to the fraction of an orally administered drug that reaches systemic circulation intact and is determined by a complex interplay of factors including drug solubility, intestinal permeability, and susceptibility to pre-systemic metabolism [86].
For many anticancer drugs, oral bioavailability is both low and highly variable, leading to unpredictable systemic exposure that can compromise therapeutic efficacy and safety [86]. This case study examines the multifaceted challenges limiting oral bioavailability of anticancer agents and systematically evaluates innovative formulation strategies, particularly advanced nanocarrier systems, that are revolutionizing oral chemotherapy. Through a comparative pharmacokinetic lens, we analyze experimental data and methodologies that demonstrate how these advanced delivery systems can overcome biological barriers while maintaining therapeutic efficacy.
Fundamental Barriers to Oral Bioavailability of Anticancer Drugs
Physicochemical and Biological Barriers
The journey of an orally administered anticancer drug through the gastrointestinal tract involves numerous challenges that collectively limit systemic bioavailability. These barriers operate sequentially and can substantially reduce the amount of active drug reaching tumor tissue.
Table 1: Primary Barriers to Oral Bioavailability of Anticancer Drugs
| Barrier Category | Specific Challenge | Impact on Bioavailability | Representative Drugs Affected |
|---|---|---|---|
| Physicochemical Properties | Low aqueous solubility | Incomplete dissolution and precipitation in GI tract | Tyrosine kinase inhibitors, Docetaxel [86] [87] |
| Chemical instability in GI environment | Degradation before absorption | Etoposide, Chlorambucil [86] | |
| Suboptimal lipid solubility | Poor permeability across enterocyte membrane | Various BCS Class III/IV drugs [86] | |
| Biological Barriers | Efflux transporters (P-gp, BCRP, MRPs) | Active transport back into gut lumen | Many tyrosine kinase inhibitors [86] |
| First-pass gut metabolism (CYP3A) | Enzymatic degradation in enterocytes | 5-fluorouracil, TKIs [86] | |
| Hepatic first-pass metabolism | Extensive processing before systemic circulation | Most orally administered anticancer drugs [85] [86] | |
| GI Environment Variability | Food effects | Altered dissolution and absorption | Drugs with fat-dependent absorption [86] |
| pH variability | Differential solubility across GI tract | Acid-labile compounds [86] | |
| Motility and transit time | Variable absorption windows | Drugs with specific absorption sites [86] |
The Biopharmaceutics Classification System categorizes drugs based on solubility and permeability characteristics, with most anticancer drugs falling into Classes II, III, or IV—all presenting challenges for oral delivery [86]. For instance, many small molecule tyrosine kinase inhibitors exhibit aqueous solubilities comparable to limestone and quartz, severely limiting their dissolution in gastrointestinal fluids [86].
Consequences of Variable Bioavailability
The clinical implications of low and variable bioavailability are substantial. Studies demonstrate that patients prescribed oral anticancer agents experience significant polypharmacy (mean of 10.9 additional medications) and drug interactions (mean of 2.1 major interactions), which further complicate bioavailability patterns [88]. Toxicity from oral anticancer agents is common, with one study reporting 80% of patients experiencing treatment-related toxicity, 36% classified as severe, and 17% requiring hospitalization within 90 days of initiation [88]. These findings underscore the critical need for formulation strategies that normalize and enhance oral bioavailability profiles.
Emerging Solutions: Advanced Nanoparticle Delivery Systems
Nanocarrier Platforms for Bioavailability Enhancement
Innovative nanoparticle-based delivery systems have emerged as promising solutions to overcome the bioavailability challenges of oral anticancer drugs. These systems protect therapeutic payloads from degradation, enhance absorption, and modulate distribution patterns.
Table 2: Nanoparticle Platforms for Oral Anticancer Drug Delivery
| Platform Type | Key Components | Mechanism of Action | Bioavailability Enhancement | Stage of Development |
|---|---|---|---|---|
| Lipid-Polymer Hybrid Nanoparticles | Biodegradable polymer core, lipid shell | Combines advantages of both systems: improved stability + enhanced permeability | Significant enhancement for various chemotherapeutics [89] | Preclinical research phase [89] |
| Polymeric Nanoparticles | PLGA, Chitosan, other natural/synthetic polymers | Drug protection, controlled release, mucoadhesion | Improves stability and absorption of encapsulated drugs [85] | Advanced preclinical studies [85] |
| Organic-Inorganic Hybrid Systems | Patented CNPharm technology | Enhanced solubility without metabolic inhibitors | 1,600x bioavailability increase for docetaxel (OTX-M) [87] | Preclinical stage [87] |
| Resveratrol Nanoformulations | Biodegradable polymers for phytochemical delivery | Protection against degradation, enhanced cellular uptake | Marked enhancement of anticancer activity [90] | Experimental models [90] |
Mechanisms of Bioavailability Enhancement
Nanoparticle systems enhance oral bioavailability through multiple complementary mechanisms. They protect encapsulated drugs from the harsh gastrointestinal environment, including acidic pH and degradative enzymes [85]. Their nanoscale size and surface modifications enhance mucus penetration and intestinal retention through mucoadhesive properties [85]. Importantly, certain nanoformulations can inhibit efflux transporters like P-glycoprotein, thereby increasing intracellular drug accumulation [85] [86]. Some advanced systems also facilitate uptake through the lymphatic system, bypassing hepatic first-pass metabolism [86].
Figure 1: Mechanism of Nanoparticle-Mediated Bioavailability Enhancement. This diagram illustrates how nanoparticle systems address multiple barriers to oral bioavailability simultaneously.
Comparative Pharmacokinetic Analysis: Oral vs. Intravenous Administration
Bioavailability and Pharmacokinetic Parameters
Direct comparison of pharmacokinetic parameters between oral and IV administration reveals both the limitations of conventional oral formulations and the potential of advanced delivery systems.
Table 3: Comparative Bioavailability of Anticancer Drugs and Analgesics (for Reference)
| Drug/Drug System | Administration Route | Absolute Bioavailability | Key Pharmacokinetic Parameters | Clinical Implications |
|---|---|---|---|---|
| Conventional Docetaxel | IV | 100% (reference) | Complete systemic availability | Requires clinic visits, infusion equipment [87] |
| OTX-M (Docetaxel Nanoformulation) | Oral | 1,600x increase vs. conventional oral | Once-daily dosing possible | Home administration, tumor inhibition rate: 82.83% [87] |
| Ibuprofen | Oral | 91% | Equivalent AUC oral vs. IV | Suitable for oral administration [46] |
| Tramadol | Oral | 80% | Higher M1 metabolite with oral route | IV dose should not be reduced when switching from oral [46] |
| Small Molecule TKIs | Oral | Highly variable (0-100%) | Erratic absorption, food effects | Requires therapeutic drug monitoring [86] |
Efficacy and Safety Outcomes
Beyond pharmacokinetic parameters, clinical efficacy and safety profiles demonstrate the potential of advanced oral formulations. For instance, the oral targeted therapy sevabertinib demonstrated a 70.5% response rate in patients with HER2-mutant non-small cell lung cancer, with manageable side effects and no reported interstitial lung disease [91]. Similarly, OTX-M achieved tumor inhibition rates up to 92.81% in preclinical models, with optimal dosing demonstrating an 82.83% inhibition rate with minimal side effects [87].
The economic implications are also substantial. Research from Rutgers University and Chung-Ang University suggests that oral anticancer therapies can reduce treatment costs by 59.9% compared to injectable chemotherapies, making them particularly valuable for resource-limited settings [87].
Experimental Protocols and Methodologies
Preclinical Evaluation of Oral Nanoformulations
Protocol 1: Bioavailability Assessment of OTX-M
- Objective: Quantify the bioavailability enhancement of organic-inorganic hybrid docetaxel formulation [87]
- Formulation: OTX-M using patented CNPharm technology without P-gp inhibitors or ritonavir [87]
- Dosing Regimen: Systematic evaluation of low (twice daily), moderate (once daily), and high (four times weekly) doses [87]
- Efficacy Endpoint: Tumor inhibition rate calculated from xenograft models [87]
- Safety Endpoint: Weight change monitoring and side effect profiling [87]
- Analytical Method: LC-MS/MS for docetaxel quantification in plasma and tissues [87]
Protocol 2: Pharmacokinetic Study of Fixed-Dose Combinations
- Design: Randomized, open-label, crossover, five-period pharmacokinetic clinical trial [46]
- Formulations: Four IV strengths of Ibuprofen/Tramadol HCl and one oral reference [46]
- Participants: Healthy volunteers (n=12) [46]
- Analytes: Enantiomers of Ibuprofen, Tramadol, and active metabolite O-desmethyl-Tramadol (M1) [46]
- Key Parameters: C~max~, AUC, absolute bioavailability calculations [46]
- Statistical Analysis: Bioequivalence testing with dose-normalized parameters [46]
Research Reagent Solutions for Oral Formulation Development
Table 4: Essential Research Reagents for Oral Anticancer Formulation Studies
| Reagent/Category | Function in Research | Specific Application Examples | Experimental Role |
|---|---|---|---|
| Biodegradable Polymers | Nanoparticle matrix formation | PLGA, Chitosan, Polymeric cores | Drug encapsulation, controlled release [89] [85] |
| Lipid Components | Hybrid nanoparticle shells | Phospholipids, triglycerides | Enhanced permeability, membrane fusion [89] |
| Organic-Inorganic Hybrid Materials | Enhanced solubility platforms | CNPharm technology | Bioavailability enhancement without metabolic inhibitors [87] |
| P-gp Inhibitors | Efflux transporter blockade | Ritonavir (conventional approach) | Increasing intracellular drug accumulation [86] |
| Analytical Standards | Drug quantification | Enantiomer-specific standards | PK parameter determination for chiral drugs [46] |
| CYP Enzyme Substrates/Inhibitors | Metabolism studies | CYP3A4 probes | First-pass metabolism assessment [86] |
Figure 2: Experimental Workflow for Oral Anticancer Formulation Development. This diagram outlines the systematic approach from formulation design through clinical translation.
Current Developments and Future Perspectives
The field of oral anticancer therapy continues to evolve rapidly. As of 2025, more than 80 oral chemotherapeutic agents have received regulatory approval in the United States and Europe, with many additional candidates in advanced development stages [85]. Recent approvals include drugs such as abemaciclib for breast cancer, osimertinib for lung cancer, and enzalutamide for prostate cancer, all offering targeted mechanisms with improved tolerability profiles [92].
The pipeline of innovative oral therapies continues to expand, with sevabertinib recently receiving FDA Breakthrough Therapy designation and priority review for HER2-mutant NSCLC [91]. Such advancements highlight the growing emphasis on targeted oral therapies that address specific molecular pathways while minimizing systemic toxicity.
Future development directions include more sophisticated nanoparticle systems with stimuli-responsive release mechanisms, enhanced tumor-specific targeting capabilities, and combination approaches that concurrently deliver multiple therapeutic agents with synergistic activities. As these technologies mature, oral chemotherapy is poised to transform cancer management into a more chronic disease model similar to hypertension or diabetes, significantly improving patient quality of life while maintaining therapeutic efficacy [87].
The case study demonstrates that while significant challenges remain in optimizing oral bioavailability of anticancer drugs, advanced formulation strategies—particularly nanoparticle-based delivery systems—offer promising solutions to these limitations. Through protection from degradation, enhanced absorption, and bypass of metabolic pathways, these systems can dramatically improve the pharmacokinetic profiles of oral anticancer agents.
The comparative analysis between oral and intravenous administration routes reveals a shifting paradigm in cancer treatment, where patient-centered oral therapies are increasingly achieving comparable efficacy to traditional IV regimens while offering substantial advantages in convenience, cost-effectiveness, and quality of life. As formulation science continues to advance, oral chemotherapy is positioned to become an increasingly dominant modality in oncology, fundamentally changing how cancer is treated and managed across healthcare systems worldwide.
Evidence and Outcomes: Clinical and Preclinical Validation of Administration Route Efficacy
The route of administration is a fundamental determinant of a drug's pharmacokinetic (PK) profile, directly influencing its onset of action, intensity of effect, and therapeutic utility. Comparative pharmacokinetics research provides the empirical foundation for selecting the appropriate administration pathway, bridging preclinical findings with clinical application. This guide objectively analyzes the key PK parameters—Area Under the Curve (AUC), maximum concentration (Cmax), and time to Cmax (Tmax)—across intravenous (IV) and oral (PO) routes, synthesizing data from recent studies to aid drug development professionals in making informed decisions.
The IV route provides a direct reference, as it offers 100% bioavailability and avoids first-pass metabolism, enabling accurate calculation of the absolute bioavailability of other routes. In contrast, the oral route is subject to variables including gastrointestinal absorption, intestinal and hepatic metabolism, and food effects, which collectively influence the rate and extent of systemic drug exposure. Quantitative comparison of AUC, Cmax, and Tmax between these routes is therefore critical for dose bridging, formulation optimization, and predicting clinical outcomes [93] [31].
Quantitative PK Parameter Comparison: Intravenous vs. Oral Administration
The following tables consolidate pharmacokinetic data from recent head-to-head studies, providing a comparative overview of systemic exposure (AUC), peak concentration (Cmax), and absorption rate (Tmax) across different drug classes.
Table 1: Comparative Pharmacokinetics of Intravenous vs. Oral Administration in Humans
| Drug | Route | Dose | AUC (h·μg/mL) | Cmax (μg/mL) | Tmax (h) | Absolute Bioavailability (F%) | Study Details |
|---|---|---|---|---|---|---|---|
| Ibuprofen [31] | IV | 400 mg | 70.72 (S-enantiomer) | 27.84 (S-enantiomer) | - | 100 (Reference) | Healthy volunteers, crossover trial |
| Oral | 400 mg | 64.91 (S-enantiomer) | 19.34 (S-enantiomer) | ~0.5-1* | ~91% | Granules for oral solution | |
| Tramadol [31] | IV | 37.5 mg | Not specified | Not specified | - | 100 (Reference) | Healthy volunteers, crossover trial |
| Oral | 37.5 mg | Not specified | Not specified | ~1-2* | ~80% | Granules for oral solution | |
| Chrysin [94] | Oral (Unformulated) | Single dose | 914.8 (ng·h/mL)* | 87.3 (ng/mL)* | Not specified | Not calculated | Healthy adults, crossover trial |
| Oral (Micellar) | Single dose | >2.6-fold vs. Unformulated | >2.6-fold vs. Unformulated | Not specified | Not calculated | Novel formulation to enhance bioavailability |
Note: *Estimated from plasma concentration-time curves; Values for ibuprofen and tramadol are geometric means; Chrysin data presented as mean ± SD.
Table 2: Comparative Pharmacokinetics in Animal Models
| Drug / Species | Route | Dose | Key PK Parameters | Absolute Bioavailability (F%) | Study Details |
|---|---|---|---|---|---|
| Meloxicam / Goats [95] | IV | 0.5 mg/kg | Terminal t1/2: Shorter in femalesMRT: Shorter in females | 100 (Reference) | Crossover design, 12 Saanen goats (6 female, 6 male) |
| Oral | 1.0 mg/kg | Terminal t1/2: Longer in malesMRT: Longer in malesAUC: Higher in males | 77.4% (Females)104.7% (Males) | Gender-based differences observed | |
| Difloxacin / Pigeons [96] | IV | 10 mg/kg | Vz: 2.52 ± 0.65 L/kgt1/2: 4.27 ± 1.14 h | 100 (Reference) | Random design, 30 pigeons (n=10 per route) |
| Oral | 10 mg/kg | Cmax: 1.81 ± 0.47 μg/mLTmax: 2.60 ± 0.97 ht1/2: 2.64 ± 0.64 h | 38.4% ± 10.5% | Considered insufficient for pathogens with MIC >0.1 μg/mL |
Key Observations from Comparative Data
AUC and Bioavailability: The AUC for oral ibuprofen is equivalent to its IV formulation, resulting in high absolute bioavailability (91%), suggesting minimal first-pass loss [31]. In contrast, oral difloxacin in pigeons shows low bioavailability (38.4%), indicating significant pre-systemic elimination or incomplete absorption [96]. Meloxicam in goats demonstrates that bioavailability can be gender-dependent, with males exhibiting unexpectedly high oral bioavailability (104.7%), potentially due to gender-based variations in hepatic metabolism or enterohepatic recirculation [95].
Cmax and Tmax: IV administration invariably results in a higher Cmax achieved almost immediately post-dose. Oral Cmax is consistently lower and occurs later (Tmax). For example, S-Ibuprofen after oral administration had a Cmax of 19.34 μg/mL, significantly lower than the IV Cmax of 27.84 μg/mL [31]. The Tmax for oral difloxacin was 2.6 hours, indicating slow absorption in pigeons [96].
Formulation Impact: Advanced formulations can dramatically alter oral PK parameters. A novel micellar chrysin formulation achieved more than a 2.6-fold increase in systemic exposure (AUC) and Cmax compared to an unformulated preparation, highlighting the critical role of formulation in overcoming poor solubility and extensive first-pass metabolism [94].
Detailed Experimental Protocols from Key Studies
To ensure the reliability and reproducibility of comparative PK data, rigorous and standardized experimental methodologies are employed. The following protocols are sourced from the studies cited in this guide.
Protocol: Ibuprofen/Tramadol IV vs. Oral Study in Humans
This randomized, open-label, crossover clinical trial directly compared the pharmacokinetics of fixed-dose combinations of Ibuprofen and Tramadol via IV and oral routes in healthy volunteers [31].
- Study Design: A five-period crossover trial. Each participant received a single dose of four different IV strengths and one oral formulation in randomized order.
- Subjects: 12 healthy subjects (7 male, 5 female). The study was conducted in compliance with ethical standards (EudraCT code: 2017-001303-77).
- Formulations and Dosing: IV formulations contained Ibuprofen (400 mg) with Tramadol HCl (30, 31.5, 33, 37.5 mg). The oral formulation was Ibuprofen/Tramadol HCl (400 mg/37.5 mg) granules for oral solution.
- Blood Sampling: Plasma samples were collected at predefined intervals post-dose to characterize the concentration-time profile fully.
- Bioanalytical Method: Enantiomers of Ibuprofen, Tramadol, and its active metabolite O-desmethyl-Tramadol (M1) were quantified. Pharmacokinetic parameters (Cmax, AUC) were estimated using non-compartmental analysis.
Protocol: Meloxicam IV vs. Oral Study in Goats
This study investigated the effect of gender on the pharmacokinetics of meloxicam in goats following IV and oral administration using a crossover design [95].
- Study Design: A crossover design consisting of two experimental phases separated by a 10-day washout period.
- Animals: 12 clinically healthy Saanen goats (six females and six males), approximately one year of age.
- Formulations and Dosing: Meloxicam was administered as an IV solution (0.5 mg/kg) and oral tablets (1.0 mg/kg). Doses were selected based on previous pharmacokinetic studies in ruminants.
- Blood Sampling: Plasma samples were collected up to 96 h post-administration at multiple time points.
- Bioanalytical Method: Plasma meloxicam concentrations were analyzed using a validated HPLC system with a diode array detector. Pharmacokinetic parameters were calculated and statistically compared between genders and administration routes.
Protocol: Difloxacin IV, IM, and Oral Study in Pigeons
This study characterized the pharmacokinetics of difloxacin in pigeons across three administration routes [96].
- Study Design: 30 pigeons were randomly divided into three groups (IV, IM, and PO; n = 10 per group).
- Dosing: Difloxacin was administered at a single dose of 10 mg/kg body weight via each route.
- Blood Sampling: Blood samples were collected serially from 0 to 48 hours post-administration.
- Bioanalytical Method: Plasma difloxacin concentrations were determined using a validated high-performance liquid chromatography (HPLC) method.
- Pharmacokinetic Analysis: Parameters were determined using Phoenix software and a non-compartmental analysis (NCA) approach.
Visualization of Workflows and Relationships
The following diagrams illustrate the core concepts and experimental workflows central to comparative pharmacokinetic studies.
PK Parameter Relationship Logic
Diagram Title: PK Parameter Relationships
Experimental Workflow for Comparative PK Studies
Diagram Title: Comparative PK Study Workflow
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Reagents and Materials for Comparative PK Studies
| Item | Function / Application | Specific Example from Literature |
|---|---|---|
| Analytical Standards | Used for calibration and quantification in bioanalytical assays; essential for method validation and ensuring accuracy. | Meloxicam and piroxicam (internal standard) analytical standards [95]. |
| HPLC System with Detector | Workhorse instrument for separating and quantifying drug concentrations in biological samples (e.g., plasma). | Agilent 1260 HPLC system with a diode array detector (DAD) [95]. |
| Validated Bioanalytical Method | A rigorously tested protocol ensuring that the assay is selective, sensitive, accurate, and precise for the analyte in a specific matrix. | A validated HPLC method for difloxacin in pigeon plasma [96]. |
| Commercial Formulations | Commercially available IV and oral drug products used to ensure clinical relevance and regulatory alignment. | IV (Metacam) and oral (Melox Fort) formulations of meloxicam [95]. |
| Pharmacokinetic Software | Used for non-compartmental analysis (NCA) or compartmental modeling to calculate PK parameters from concentration-time data. | Phoenix software for NCA of difloxacin data [96]. |
Direct, head-to-head comparison of pharmacokinetic parameters across administration routes provides indispensable data for rational drug development. The evidence consolidated in this guide confirms that the IV route offers complete bioavailability and immediate systemic exposure, serving as the benchmark for assessing other routes. The oral route, while convenient, is subject to significant variability influenced by factors such as a drug's physicochemical properties, formulation, and interspecies—as well as intraspecies—physiological differences, including gender.
Researchers must therefore base critical decisions—such as lead compound selection, formulation strategy, and dose regimen projection from animals to humans—on robust, route-specific PK data. The quantitative tables, detailed protocols, and research tools provided here aim to serve as a practical resource for designing and interpreting such essential comparative studies.
The route of drug administration—specifically, the choice between oral and intravenous (IV) delivery—directly dictates a drug's pharmacokinetic (PK) profile, which in turn fundamentally shapes its therapeutic efficacy and safety. This comparison guide examines the core PK parameters of bioavailability, concentration-time profiles, and clearance that differ between these routes. By integrating quantitative data from clinical and preclinical studies, this analysis demonstrates how these differences correlate with clinical outcomes, influence the risk of adverse events, and guide the strategic application of IV to oral switch therapy. The establishment of a clear PK/PD relationship is essential for optimizing drug regimens across diverse therapeutic areas.
The path a drug takes through the body could not be more different between oral and intravenous administration. Intravenous administration delivers a drug directly into the systemic circulation, achieving a 100% bioavailability and immediate high plasma concentrations [97]. In contrast, oral administration introduces a drug via the gastrointestinal tract, where it must be absorbed and, for many compounds, survive first-pass metabolism in the liver, resulting in reduced and delayed systemic availability [97]. These fundamental differences in the journey to the target site create distinct concentration-time profiles that form the basis for all subsequent therapeutic and safety considerations.
Understanding these pharmacokinetic (PK) differences is not an academic exercise; it is a clinical necessity for personalized therapy and risk assessment. The concentration-time profile of a drug determines the speed of onset of action, the intensity of the pharmacological effect, and the duration of therapeutic coverage. Furthermore, the relationship between a drug's plasma concentration and its desired and undesired effects—its pharmacodynamics (PD)—is paramount for designing rational dosing regimens [98] [99]. This guide provides a structured comparison of oral and IV routes, linking their distinct PK profiles to documented therapeutic outcomes and safety profiles to inform researchers and drug development professionals.
Quantitative PK Parameter Comparison: Oral vs. IV Delivery
The divergence in the pharmacokinetic profiles of oral versus IV administration can be quantified through several key parameters. The table below summarizes the core differences that define these two routes.
Table 1: Core Pharmacokinetic Parameter Comparison: Oral vs. Intravenous Administration
| PK Parameter | Oral Administration | Intravenous Administration | Clinical Correlation |
|---|---|---|---|
| Bioavailability (F) | Variable; often <100% due to first-pass metabolism and absorption limitations [97] | 100% (gold standard) [97] | Determines the required dose to achieve equivalent systemic exposure. |
| Time to Peak (Tmax) | Delayed; depends on gastric emptying and absorption rate [97] | Immediate (end of infusion) [97] | Dictates the speed of onset of pharmacological effect. |
| Peak Concentration (Cmax) | Generally lower for the same total dose [100] | High, achieved immediately [100] | High Cmax may be linked to acute toxicity (e.g., CNS effects with ST-246 [100]); low Cmax may risk inefficacy. |
| Absorption Phase | Required; subject to inter- and intra-patient variability [97] | Not applicable | Eliminates variability from absorption, ensuring predictable exposure. |
Beyond these fundamental parameters, the volume of distribution (Vd) and clearance (CL) are critical for determining dosing frequency and steady-state concentrations. While these parameters are intrinsic to the drug itself and should, in theory, be route-independent, the administration route can influence them in practice. For instance, saturation of distribution or elimination pathways has been observed with IV administration due to high initial concentrations, leading to non-linear kinetics, as was the case with high-dose IV ST-246 in non-human primates [100].
Table 2: Impact of Route on Broader Dosing and Safety Considerations
| Consideration | Oral Administration | Intravenous Administration |
|---|---|---|
| Dosing Regimen | Often suitable for outpatient and chronic therapy. | Typically requires inpatient care and professional administration. |
| Cost | Lower drug and administration costs [64]. | Higher costs (drug, diluents, equipment, nursing time) [64]. |
| Safety & Tolerability | Avoids IV-related risks (infection, phlebitis); slower onset may prevent acute Cmax-driven toxicity [64] [100]. | Carries risk of infusion-related reactions, infection, and phlebitis; potential for acute, concentration-dependent toxicity [64] [100]. |
| Flexibility | Allows for easy IV-to-oral switch therapy (sequential, switch, or step-down) [64]. | Necessary for critically ill, nauseated, or peri-operative patients. |
Clinical Correlations: Therapeutic Outcomes and Safety Profiles
Case Study: ST-246 (Tecovirimat) – Managing Cmax-Driven Toxicity
The development of an IV formulation for the antiviral ST-246 provides a compelling case study linking PK to safety. Oral ST-246 has demonstrated excellent safety and efficacy. However, short IV infusions in animal models resulted in dose-limiting central nervous system (CNS) effects that were directly correlated with high peak plasma concentrations (Cmax) [100]. This acute toxicity was not observed with oral administration, which produces a slower absorption phase and a lower Cmax for the same total exposure (AUC).
Experimental Protocol: The safety and PK of IV ST-246 were evaluated in mice, rabbits, and non-human primates (NHP). The drug was administered via short IV infusions (e.g., 10 minutes in mice, 4-6 hours in NHP) at various doses. Plasma concentrations were monitored, and animals were closely observed for adverse events [100].
Correlation and Outcome: The study found that high Cmax levels from rapid IV infusion were associated with adverse CNS events. By modifying the IV administration to a slower, prolonged infusion, researchers successfully lowered the Cmax while maintaining the necessary total drug exposure (AUC). This strategy effectively mitigated the toxicity, demonstrating that controlling the rate of administration and the resulting PK profile is as critical as the total dose for ensuring safety [100].
IV-to-Oral Switch Therapy: A Widespread Clinical Application
The strategic transition from IV to oral therapy is a well-established practice to leverage the advantages of each route while mitigating their disadvantages. The primary PK parameter enabling this switch is the bioavailability of the oral formulation. If bioavailability is sufficient (often >50-60%), oral therapy can maintain therapeutic systemic concentrations achieved by the initial IV loading dose [64].
Patient Selection Protocol: The criteria for considering an IV-to-oral switch include [64]:
- Hemodynamic stability and afebrile status for >24 hours.
- Resolution of clinical signs and symptoms of infection.
- Normalizing white blood cell count.
- Ability to ingest and absorb oral medications.
- Improvement in signs and symptoms of infection.
Therapeutic and Economic Outcomes: Adhering to this protocol has demonstrated significant benefits. Studies have shown that early IV-to-oral conversion is associated with reduced hospital length of stay, lower drug acquisition costs, and elimination of IV-related complications (e.g., cannula-related infections and thrombophlebitis), all without compromising clinical cure rates [64]. This practice exemplifies how understanding comparative pharmacokinetics leads to improved patient outcomes and more efficient healthcare delivery.
Pharmacodynamics and Safety: The Example of QT Prolongation
The relationship between PK and pharmacodynamics (PD) is critical for safety assessment, particularly for concentration-dependent adverse effects like QT interval prolongation. The free (unbound) plasma concentration of a drug is often predictive of its effect on cardiac ion channels, such as the hERG potassium channel [98].
Correlation and Risk Assessment: For drugs that inhibit the hERG channel, the free plasma concentration associated with QT prolongation in clinical studies often correlates with the concentration causing inhibition in vitro. This allows for a quantitative safety assessment during drug development. A common strategy is to ensure a substantial safety multiple (e.g., 30-fold) exists between the therapeutic free plasma concentration and the concentration associated with QT prolongation [98]. This PK/PD analysis is route-independent but is fundamental for setting safe exposure limits for both IV and oral formulations.
Visualizing the PK/PD Pathway and Experimental Workflow
Diagram 1: PK/PD Pathway
Diagram 2: Experimental PK Workflow
Table 3: Essential Research Reagents and Tools for Comparative PK Studies
| Tool/Reagent | Function in PK Research | Application Example |
|---|---|---|
| Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS) | A highly sensitive and specific bioanalytical technique for quantifying drug and metabolite concentrations in biological matrices like plasma [101] [99]. | Used to determine piperacillin concentrations in patient plasma for therapeutic drug monitoring (TDM) [101]. |
| Physiologically Based Pharmacokinetic (PBPK) Modeling Software | In silico platforms that simulate ADME processes to predict drug concentration-time profiles in virtual populations, aiding in study design and DDI prediction [102]. | Used to model complex drug-drug interactions (DDIs), such as enzyme induction by rifampicin [102]. |
| Population PK Software (e.g., MwPharm, TDMx, ID-ODs) | Software that utilizes Bayesian forecasting to estimate individual patient PK parameters using sparse concentration data and a prior population model [101]. | Employed for model-informed precision dosing of antibiotics like piperacillin in critically ill patients [101]. |
| Stable Isotope-Labeled Internal Standards | Compounds identical to the analyte but with a different isotopic mass; used in LC-MS/MS to correct for variability in sample preparation and ionization efficiency [101]. | Included in the bioanalysis of piperacillin to improve assay accuracy and precision [101]. |
| In Vitro Transwell Systems (e.g., Caco-2 cell models) | Cell-based assays used to predict a drug's intestinal permeability and absorption potential in humans [99]. | A standard tool in early drug discovery to screen compounds for oral bioavailability. |
| Human Liver Microsomes / Hepatocytes | In vitro systems containing drug-metabolizing enzymes (CYPs, UGTs) used to study a drug's metabolic stability and identify metabolites [99]. | Used to determine metabolic clearance and to assess the potential for metabolic drug-drug interactions. |
The comparative analysis of oral and intravenous pharmacokinetics reveals a consistent narrative: the route of administration is a critical determinant of clinical utility. The IV route offers complete bioavailability and immediate effect, which is indispensable in emergency settings, but it carries inherent risks related to high Cmax and requires significant healthcare resources. The oral route, while subject to the variables of absorption and first-pass metabolism, provides a safer, more convenient, and cost-effective option for sustained therapy. The practice of IV-to-oral switch therapy successfully bridges these two worlds, leveraging the initial control of IV to establish efficacy before transitioning to the practical benefits of oral dosing. For researchers and drug developers, a deep understanding of these PK/PD relationships, supported by robust bioanalytical and modeling tools, is fundamental to designing optimal, safe, and effective drug regimens from discovery through to patient care.
The selection of an appropriate administration route is a pivotal decision in drug development, directly influencing a compound's bioavailability, therapeutic efficacy, and safety profile. Preclinical models serve as the indispensable bridge between in vitro assays and human clinical trials, providing critical insights into how a drug's journey—from administration site to systemic circulation—is affected by the chosen route. Within the context of comparative pharmacokinetics of oral versus intravenous administration, these models help elucidate complex interactions involving first-pass metabolism, route-dependent gut metabolism, and enterohepatic circulation [103]. The fidelity of these models in predicting human outcomes is paramount; however, even the most advanced models face challenges such as interspecies differences and the inability to fully replicate human pathological conditions [104] [105]. A rigorous, model-informed approach in the preclinical stage is therefore essential to de-risk clinical translation and optimize the probability of success for new therapeutic entities [106].
Preclinical models can be broadly categorized, each with distinct advantages and limitations for evaluating administration routes. The selection of a model is critical and should be guided by factors including physiological similarity, the research question, and the drug's mechanism of action [104].
Table 1: Classification and Application of Preclinical Models in Route-Dependent Studies
| Model Category | Key Characteristics | Utility in Oral/IV PK Studies | Key Limitations |
|---|---|---|---|
| Inbred Strains | Genetically homogeneous animals achieved through repeated generations of sibling mating. | Reduces inter-animal variability in PK parameters, useful for precise bioavailability studies. | Limited genetic diversity may not reflect variability in human populations [104]. |
| Disease Induction | Disease state is experimentally induced (e.g., via chemicals, surgery, or infection). | Allows study of how disease pathophysiology (e.g., gut inflammation, liver impairment) alters route-dependent PK. | The induced condition may not fully mimic the natural progression of human disease [104] [107]. |
| Xenograft | Human cells or tissues are transplanted into immunodeficient animals. | Primarily used in oncology to test drug efficacy; can be used to compare tumor drug levels after oral vs IV dosing. | Lack of intact immune system and human-like tumor microenvironment [104]. |
| Genetically Engineered | Genetic composition is altered (e.g., gene knockout, knock-in, humanization). | "Humanized" models expressing human enzymes/transporters are invaluable for predicting human-first pass metabolism [104]. | High cost and technical complexity; may have unforeseen phenotypic consequences. |
| Spontaneous/Natural Occurrence | Animals that naturally develop a disease without deliberate manipulation. | Provides a naturally occurring disease context to study drug absorption and disposition [104]. | Limited availability and control over disease onset and progression. |
| Bioengineered Human Models (Organoids, Organs-on-Chips) | 3D structures derived from human stem cells or microfluidic devices mimicking human organ physiology. | Can model human-specific gut and liver metabolism, bypassing interspecies differences [105]. | Still emerging technology; may not fully capture systemic integration and immune responses [105]. |
Quantitative Data from Route Comparison Studies
Clinical and preclinical pharmacokinetic studies provide the quantitative foundation for comparing administration routes. The data below, derived from a clinical study on a Tramadol/Ibuprofen combination, exemplifies key PK parameters that are typically characterized in preclinical settings to bridge towards human doses [3].
Table 2: Pharmacokinetic Comparison of Oral vs. Intravenous Administration of Ibuprofen and Tramadol [3]
| Analyte | Administration Route | Absolute Bioavailability | Key PK Parameter Comparisons | Clinical Interpretation |
|---|---|---|---|---|
| Ibuprofen | Oral (400 mg) | 91% | Equivalent AUC; lower Cmax and delayed Tmax vs. IV. | Oral route provides equivalent overall exposure (AUC) but slower onset. |
| Tramadol | Oral (37.5 mg) | 80% | Higher Cmax and AUClast for parent drug with IV; metabolite (M1) patterns differ. | IV administration delivers parent drug more efficiently; oral route undergoes significant first-pass metabolism. |
The data in Table 2 highlights critical route-dependent phenomena. The high oral bioavailability of Ibuprofen (91%) suggests minimal first-pass loss, whereas Tramadol's lower bioavailability (80%) and the different metabolite profile indicate significant pre-systemic metabolism [3]. These findings underscore why understanding route-dependent phase-II gut metabolism and enterohepatic circulation, as investigated with resveratrol, is vital for accurate prediction [103]. Intravenous administration, by guaranteeing complete absorption, serves as the reference point for calculating the absolute bioavailability of other routes [108].
Experimental Protocols for Route-Dependent PK Studies
A robust preclinical pharmacokinetic study requires meticulous design and execution. The following protocol outlines a standardized crossover study to compare oral and intravenous administration.
Protocol: Preclinical Crossover Study for Oral vs. IV Pharmacokinetics
- Study Design: A randomized, open-label, crossover design is recommended. Each subject receives both the oral and IV formulation of the drug in separate periods, with a sufficient washout period in between to prevent carry-over effects [3].
- Formulations:
- IV Solution: A sterile solution for intravenous bolus or infusion. The formulation should ensure solubility and stability.
- Oral Formulation: Can be a solution, suspension, or granules in water, dosed via oral gavage to ensure accurate delivery [3].
- Blood Sampling Schedule: Serial blood samples are collected pre-dose and at multiple time points post-dose (e.g., 5, 15, 30 min and 1, 2, 4, 8, 12, 24 hours). The schedule should be dense enough to accurately characterize the absorption and elimination phases [108].
- Bioanalytical Method: Plasma concentrations of the drug and its major metabolites are quantified using a validated method, such as high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS). The method must be validated for specificity, accuracy, precision, and lower limit of quantification [108].
- Pharmacokinetic Analysis: Non-compartmental analysis (NCA) is performed to estimate key parameters [108]:
- AUC0-t and AUC0-∞: Area under the concentration-time curve, indicating total systemic exposure.
- Cmax: Maximum observed plasma concentration.
- Tmax: Time to reach Cmax.
- t1/2: Elimination half-life.
- Absolute Bioavailability (F): Calculated as (AUCoral / Doseoral) / (AUCIV / DoseIV) × 100%.
- Statistical Analysis: An analysis of variance (ANOVA) is typically performed on log-transformed AUC and Cmax data to evaluate the bioequivalence or differences between routes [108].
Visualizing the Translation Path from Model to Human
The PATH (Preclinical Assessment for Translation to Humans) framework provides a structured approach to evaluate the strength of evidence supporting the translation of preclinical findings, such as route-dependent efficacy, to human trials [106]. The following diagram maps this logical pathway.
Diagram: PATH Framework for Route Translation
The Scientist's Toolkit: Essential Research Reagents and Materials
The following table details key reagents and materials essential for conducting high-quality preclinical pharmacokinetic studies focused on route administration.
Table 3: Essential Research Reagent Solutions for Preclinical PK Studies
| Item | Function/Application | Specific Use in Oral/IV Studies |
|---|---|---|
| IV Formulation Vehicles | Solubilize the drug candidate for intravenous injection, ensuring stability and sterility. | Creates a reference formulation for determining absolute bioavailability; must be compatible with IV administration [3]. |
| Oral Dosing Vehicles (e.g., CMC, PEG) | Suspend or solubilize the drug for accurate oral gavage dosing. | Ensures consistent and accurate delivery of the oral dose; the vehicle should not significantly alter absorption or metabolism [3]. |
| HPLC-MS/MS Systems | High-sensitivity analytical instrumentation for quantifying drug and metabolite concentrations in biological matrices. | The gold standard for generating reliable concentration-time data from plasma samples for PK parameter calculation [108]. |
| Stable Isotope-Labeled Internal Standards | Used in mass spectrometry to correct for matrix effects and variability in sample preparation. | Critical for achieving accurate and precise quantification of drug and metabolite levels in complex biological samples like plasma [108]. |
| Enzyme-Specific Probe Substrates & Inhibitors | Investigate the role of specific metabolic enzymes (e.g., CYPs, UGTs) in drug disposition. | Used in in vitro assays with liver microsomes or hepatocytes to predict first-pass metabolism potential for an oral drug [103]. |
| Humanized Animal Models | Genetically engineered models expressing human drug-metabolizing enzymes or transporters. | Provide a more predictive system for studying human-relevant gut and hepatic first-pass metabolism after oral dosing [104]. |
The strategic evaluation of administration routes through sophisticated preclinical models is a cornerstone of rational drug development. By leveraging a combination of traditional animal models, emerging bioengineered human systems, and rigorous pharmacokinetic protocols, researchers can generate robust data on route-dependent efficacy and bioavailability. Framing this work within structured approaches like the PATH framework helps systematically assess the strength of evidence for clinical translation. As the field evolves, the increased use of humanized and complex in vitro models promises to enhance the predictive power of preclinical studies, ultimately leading to more efficient and successful development of therapeutics administered via the optimal route.
The management of bone and joint infections (BJIs) has been historically dominated by the use of prolonged intravenous (IV) antibiotic therapy, based on theoretical concerns regarding adequate bone penetration and sustained bactericidal concentrations [109]. This conventional approach generates substantial healthcare expenditures, extended hospitalization, vascular access complications, and diminished patient quality of life [109]. However, recent high-quality evidence has challenged this decades-old paradigm, demonstrating that orally administered antibiotics can achieve serum and bone concentrations comparable to their intravenous counterparts due to excellent bioavailability of several antimicrobial agents [109] [50].
This case study examines the comparative efficacy of IV versus oral antibiotic regimens for bone infections through the lens of a specific clinical model, contextualizing findings within the broader research on comparative pharmacokinetics of oral versus intravenous administration. We synthesize evidence from pivotal randomized controlled trials, real-world implementation studies, and meta-analyses to provide a comprehensive, data-driven comparison for researchers, scientists, and drug development professionals working in antimicrobial therapy and infectious disease pharmacology.
Theoretical Foundations: Pharmacokinetic Principles of Antibiotic Administration
Bioavailability Considerations in Systemic Infection
The fundamental pharmacokinetic principle governing route of administration is bioavailability, defined as the fraction of an administered drug that reaches systemic circulation unchanged. While IV administration guarantees 100% bioavailability, several oral antibiotics approach near-equivalent bioavailability ( Table 1 ).
Table 1: Bioavailability Profiles of Common Antibiotics Used in Bone and Joint Infections
| Antibiotic Class | Example Agents | Oral Bioavailability | PK/PD Considerations for Bone Infection |
|---|---|---|---|
| Fluoroquinolones | Ciprofloxacin, Moxifloxacin | 70-90% [82] | Excellent bone penetration; concentration-dependent killing |
| Lincomycins | Clindamycin | ~90% [82] | Good bone penetration; time-dependent killing |
| Azoles | Fluconazole | ~90% [82] | Good bone penetration; concentration-dependent killing |
| Oxazolidinones | Linezolid | ~100% | Good bone penetration; time-dependent killing |
| Penicillins | Amoxicillin, Flucloxacillin | 50-90% [82] | Variable bone penetration; time-dependent killing |
| Cephalosporins | Cefalexin | 50-90% [82] | Variable bone penetration; time-dependent killing |
A critical consideration in the initial phase of systemic infection is whether pathophysiological changes (e.g., altered gastrointestinal perfusion, systemic inflammation) might impair oral antibiotic absorption. Current evidence, though limited, suggests that bioavailability remains largely unaltered during the acute phase of infection in non-critically ill patients [110]. Systematic review of pharmacokinetic studies has identified a significant knowledge gap in this area, though available data on ciprofloxacin and clarithromycin indicate no clinically relevant reduction in bioavailability during febrile illness [110].
Tissue Penetration and Bone Bioavailability
Achieving adequate antibiotic concentrations at the site of infection is paramount for effective treatment of osteomyelitis. The key determinants of bone penetration include:
- Lipid solubility: Highly lipophilic drugs (e.g., fluoroquinolones, clindamycin, linezolid) generally demonstrate superior bone penetration
- Protein binding: Highly protein-bound drugs have reduced free fraction available for tissue distribution
- Molecular size: Smaller molecules diffuse more readily into bone tissue
- Inflammation state: Enhanced penetration may occur during acute inflammation due to increased blood flow and capillary permeability
Antibiotics with documented excellent bone penetration include fluoroquinolones, linezolid, and certain cephalosporins, which achieve synovial and bone concentrations often exceeding the minimum inhibitory concentrations (MICs) of common pathogens [109].
Methodological Framework: Experimental and Clinical Trial Designs
Pivotal Clinical Trial Protocols
The landmark Oral Versus Intravenous Antibiotics (OVIVA) trial established the contemporary evidence base for oral antibiotic therapy in BJIs [111] [112]. The experimental design elements of this and other key studies provide methodological templates for comparative efficacy research.
Table 2: Key Methodological Elements of Pivotal Comparative Studies
| Study Element | OVIVA Trial [111] [112] | RNOH Validation Study [111] | 2025 Meta-Analysis [113] [109] |
|---|---|---|---|
| Design | Multicenter, randomized, non-inferiority trial | Retrospective cohort study | Systematic review and meta-analysis |
| Participants | 1,054 adults with bone/joint infection | 121 patients with BJIs | 1,723 patients from 9 RCTs |
| Intervention | Early switch to oral antibiotics (within 7 days) | OVIVA protocol implementation | Oral vs. IV antibiotics across multiple trials |
| Comparison | Continued IV antibiotics for ≥6 weeks | Historical controls | IV antibiotic regimens |
| Primary Outcome | Definitive treatment failure within 1 year | Definite treatment failure at 1 year | Treatment failure, adverse events, recurrence |
| Follow-up Duration | 1 year | 1 year | Variable by included trial |
Experimental Workflow for Comparative Studies
The following diagram illustrates the standardized experimental workflow for comparative efficacy studies of IV versus oral antibiotics in bone infection models:
Diagram 1: Experimental workflow for comparative antibiotic studies
Comparative Outcomes Analysis
Efficacy Endpoints
The primary measure of comparative efficacy across studies is definitive treatment failure, typically defined as the recurrence of infection within one year of treatment initiation. Secondary efficacy measures include need for additional surgical interventions, microbiological persistence, and radiological progression.
Table 3: Comparative Efficacy Outcomes from Key Studies
| Study | Oral Antibiotic Failure Rate | IV Antibiotic Failure Rate | Statistical Significance | Non-inferiority Margin |
|---|---|---|---|---|
| OVIVA Trial (2019) | 13.2% (67/509) | 14.6% (74/506) | p<0.001 for non-inferiority | 7.5 percentage points |
| Herlev Implementation (2025) | 13% (16/127) | N/A (single-arm) | N/A | N/A |
| 2025 Meta-Analysis (9 RCTs) | RR 0.96 (95% CI 0.78-1.17) | Reference | p=0.68 | Established |
| RNOH Cohort | Comparable to historical IV controls | Historical control data | Non-inferior | N/A |
Subgroup analyses from the 2025 meta-analysis revealed important nuances in comparative efficacy. While overall recurrence rates were similar (RR 0.96; 95% CI 0.78-1.17; p=0.68), sensitivity analysis restricted to osteomyelitis and joint infections (excluding fracture-related infections) demonstrated significantly lower recurrence with IV therapy (RR 1.47; 95% CI 1.08-2.02; p=0.02) [113] [109].
Safety and Tolerability Profile
Adverse event profiles differ significantly between administration routes, with each demonstrating distinct patterns of complications:
Table 4: Comparative Safety and Adverse Event Profiles
| Safety Parameter | Oral Antibiotics | IV Antibiotics | Clinical Implications |
|---|---|---|---|
| Serious Adverse Events | 13.2-36% [111] | 14.6% [112] | Comparable frequency |
| Gastrointestinal Effects | Most common ADR (36% [111]) | Less frequent | Dose modification often required |
| IV Line Complications | Not applicable | 9.37% [112] | Significant morbidity source |
| Pharmacoeconomic Impact | £2,740 saving per patient [112] | Higher costs | Substantial system savings |
The most frequently reported adverse drug reactions with oral antibiotics were gastrointestinal (36%), followed by neuromuscular, biochemical/hematological, immunological, or microbiological manifestations [111]. IV therapy was associated with significantly higher rates of line-related complications (9.37% versus 0.96% in oral group) [112].
Clinical Decision-Making Framework
The following clinical decision pathway synthesizes evidence-based criteria for selecting appropriate patients for oral antibiotic therapy:
Diagram 2: Clinical decision pathway for antibiotic route selection
Essential Research Reagents and Methodological Solutions
Table 5: Research Reagent Solutions for Bone Infection Studies
| Reagent/Category | Specific Examples | Research Application | Experimental Function |
|---|---|---|---|
| Antibiotic-eluting Bone Graft | Cerament G (gentamicin), Cerament V (vancomycin) [111] | Surgical infection management | Local antibiotic delivery + bone void filler |
| Bioavailable Oral Antibiotics | Penicillins (68%), Fluoroquinolones, Clindamycin, Linezolid [111] | Oral treatment arm | Systemic antimicrobial activity |
| IV Antibiotic Comparators | Glycopeptides, Penicillin G, Cloxacillin [111] | IV control arm | Reference standard therapy |
| Microbiological Media | Blood culture bottles, Selective agar plates | Pathogen identification | Isolation and susceptibility testing |
| Pharmacokinetic Assays | HPLC, Mass spectrometry | Bioavailability studies | Drug concentration measurement |
| Surgical Materials | Deep bone/tissue sampling kits, Irrigation systems | Standardized surgical protocol | Source control and specimen collection |
Discussion and Research Implications
Therapeutic Equivalence and Clinical Applications
The collective evidence from randomized trials, real-world implementation studies, and meta-analyses consistently demonstrates that appropriately selected oral antibiotic regimens achieve therapeutic equivalence to IV therapy for most bone and joint infections [113] [109] [50]. The non-inferiority of oral antibiotics challenges long-standing treatment paradigms and presents opportunities to reduce healthcare costs, improve patient quality of life, and enhance antimicrobial stewardship.
The 2025 meta-analysis of nine randomized controlled trials (1,723 patients) confirmed comparable efficacy between routes of administration, with no significant differences in treatment failure (RR 0.96; 95% CI 0.78-1.17; p=0.68) or adverse events (RR 1.00; 95% CI 0.90-1.12; p=0.94) [113] [109]. Importantly, this comprehensive analysis incorporated two recent studies, strengthening the evidence base for oral therapeutic strategies.
Antimicrobial Stewardship and Health System Impact
Transitioning to oral antibiotic regimens for bone infections yields substantial benefits beyond clinical efficacy:
- Economic impact: Oral therapy generates significant cost savings (£2,740 per patient in the OVIVA trial) without compromising quality-adjusted life-years [112]
- Antimicrobial stewardship: Reduced utilization of IV antibiotics supports broader efforts to combat antimicrobial resistance by decreasing selective pressure from broad-spectrum agents [112]
- Healthcare utilization: Early transition to oral therapy facilitates shorter hospital stays and reduces complications associated with prolonged IV access [82] [50]
- Environmental impact: Oral treatment generates a lower carbon footprint compared to IV administration systems [50]
Limitations and Special Considerations
Despite robust evidence supporting oral antibiotic therapy, several important limitations and special considerations merit attention:
- Pathogen-specific considerations: Gram-negative infections remain under-represented in current trials [82]
- Bioavailability limitations: Not all antibiotics possess sufficient oral bioavailability or bone penetration for reliable efficacy
- Surgical considerations: Most study participants underwent adequate surgical source control, which may be prerequisite for oral therapy success [111]
- Monitoring requirements: Oral therapy still necessitates careful monitoring for adverse effects and therapeutic failure [82]
This case study demonstrates that oral antibiotics are non-inferior to IV regimens for treating bone and joint infections when appropriate patient selection, surgical management, and antimicrobial selection criteria are applied. The cumulative evidence from randomized trials, real-world implementation studies, and meta-analyses supports a paradigm shift toward oral therapy as a safe, effective, and cost-efficient approach for most patients with BJIs.
Future research should focus on optimizing oral regimens for specific pathogens, defining the role of therapeutic drug monitoring, and developing personalized approaches based on host and pathogen characteristics. For drug development professionals, these findings highlight the importance of pursuing compounds with excellent oral bioavailability and favorable bone penetration characteristics to expand future therapeutic options for serious orthopedic infections.
The selection of a drug's administration route is a critical determinant of its therapeutic success, influencing onset of action, efficacy, and safety profile. Pharmacokinetics—the study of how a drug moves through the body—encompasses the fundamental processes of absorption, distribution, metabolism, and excretion (ADME). Each administration route creates a distinct ADME profile, necessitating careful evaluation during drug development and regulatory review [22]. Intravenous (IV) administration delivers drugs directly into the systemic circulation, ensuring complete bioavailability and immediate therapeutic effects. In contrast, oral (PO) administration requires the drug to navigate the gastrointestinal tract and hepatic portal system, introducing variables that can reduce bioavailability through first-pass metabolism and other barriers [22]. This guide objectively compares the pharmacokinetic performance of intravenous versus oral administration, providing experimental data and methodologies to inform regulatory and clinical decision-making for drug development professionals.
Quantitative Pharmacokinetic Comparison
The differences between intravenous and oral administration can be quantified through key pharmacokinetic parameters. The table below summarizes comparative data from clinical and preclinical studies.
Table 1: Comparative Pharmacokinetic Parameters for Intravenous vs. Oral Administration
| Drug Compound | Administration Route | Peak Serum Concentration (µg/mL) | Time to Peak (hours) | Half-Life (hours) | Bioavailability | Key Study Findings |
|---|---|---|---|---|---|---|
| Chloramphenicol [19] | Intravenous (IV) | 15.0 | 0.75 | 4.0 | 100% (by definition) | Rapid achievement of therapeutic levels |
| Chloramphenicol [19] | Oral (PO) | 18.5 | 2-3 | 6.5 | Not fully quantified | Longer half-life, potential for drug accumulation |
| Topotecan (Lactone form) [75] | Intravenous (IV) | Not specified | Not specified | Not specified | 100% (by definition) | Rapid clearance from plasma |
| Topotecan (Lactone form) [75] | Oral (PO) | Not specified | Not specified | Not specified | 17.61% - 22.43% | Low bioavailability due to first-pass effect |
| Topotecan (Lactone form) [75] | Subcutaneous (SC) | Not specified | Not specified | Not specified | 88.05% | Sustained release profile, higher exposure |
Analysis of Comparative Data
The data reveals fundamental pharmacokinetic differences between administration routes. Intravenous administration provides precise control over drug concentrations in the bloodstream, making it indispensable for emergency medicine and critical care where immediate effect is required [22]. The direct entry into systemic circulation avoids the hepatic first-pass effect, ensuring the entire dose reaches the bloodstream in active form [22]. Oral administration, while more convenient for chronic therapy, exhibits variable absorption and typically lower bioavailability, as evidenced by topotecan's oral bioavailability of less than 25% compared to intravenous administration [75]. Furthermore, the chloramphenicol study demonstrates that oral administration can unexpectedly alter elimination kinetics, resulting in a significantly longer half-life (6.5 hours oral vs. 4.0 hours IV) that may necessitate therapeutic drug monitoring to prevent accumulation [19].
Experimental Protocols for Route Comparison
Robust pharmacokinetic comparison requires standardized experimental methodologies. Below are detailed protocols for generating comparative data.
Clinical Pharmacokinetic Study Protocol
Objective: To compare the pharmacokinetics of a drug following intravenous and oral administration in human subjects.
Methodology:
- Study Design: Utilize a randomized, crossover design with adequate washout period between administrations to eliminate carryover effects.
- Dosing: Administer the drug intravenously (e.g., 100 mg/kg/day in divided doses) for 4-5 days, followed by oral administration at the same dose after a washout period [19].
- Sample Collection: Collect multiple blood samples at predetermined time points (e.g., pre-dose, 0.25, 0.5, 1, 2, 4, 6, 8, 12, and 24 hours post-dose) after both IV and oral administrations [19].
- Biofluid Analysis: Measure drug concentrations in plasma using validated analytical methods such as UPLC-ESI-MS/MS for precision and sensitivity [75].
- CSF Sampling: For central nervous system infections, collect cerebrospinal fluid (CSF) samples at trough concentrations (e.g., 6 hours post-dose) to assess penetration into target tissues [19].
- Data Analysis: Calculate pharmacokinetic parameters including C~max~, T~max~, AUC~0-∞~, t~1/2~, and clearance using non-compartmental methods.
Preclinical Multi-Route Pharmacokinetic Study
Objective: To evaluate the pharmacokinetic profiles of different administration routes in animal models.
Methodology:
- Animal Grouping: Assign animals (e.g., rats, n=5 per group) to receive a single dose of the drug (e.g., 4 mg/kg) via intravenous, oral, and subcutaneous routes [75].
- Sample Collection: Collect blood samples at serial time points, along with urine and feces over 24 hours to determine excretion pathways [75].
- Form-Specific Analysis: For drugs existing in multiple forms (e.g., topotecan lactone and carboxylated forms), quantify each form separately using stabilized extraction methods to prevent interconversion during analysis [75].
- Tissue Distribution: At terminal time points, harvest key tissues (liver, kidney, spleen, etc.) to assess route-dependent distribution patterns [114].
- Data Processing: Employ mechanistic modeling approaches such as Physiologically Based Pharmacokinetic (PBPK) modeling to extrapolate findings across routes and species [114].
Visualization of Experimental Workflows
The following diagrams illustrate key experimental designs and decision-making processes for comparative route evaluation.
Pharmacokinetic Comparison Workflow
Clinical Decision Pathway
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 2: Key Research Reagents and Materials for Pharmacokinetic Studies
| Item | Function/Application | Specific Examples |
|---|---|---|
| UPLC-ESI-MS/MS System | High-sensitivity quantification of drugs and metabolites in biological samples | Topotecan lactone and carboxylated forms analysis [75] |
| Validated Bioanalytical Methods | Ensure accuracy, precision, and reproducibility of concentration measurements | FDA-compliant validation guidelines [75] |
| Physiologically Based Pharmacokinetic (PBPK) Modeling Software | Mechanistic modeling of ADME processes across routes | Nano-iPBPK for nanoparticle pharmacokinetics [114] |
| Berkeley Madonna Modeling Software | Model development, fitting, and visualization | Interactive pharmacokinetic-pharmacodynamic simulations [115] |
| Stabilized Collection Tubes | Preserve chemical form integrity during sample processing | Tubes with lactone ring stabilization buffers [75] |
Regulatory and Clinical Implications
The interpretation of comparative route data carries significant implications for drug development and therapeutic use. Regulatory agencies require comprehensive route-specific pharmacokinetic data to establish appropriate dosing regimens, especially when transitioning from IV to oral formulations during treatment [19]. The finding that oral chloramphenicol produces higher trough CSF levels (6.6 µg/mL oral vs. 4.2 µg/mL IV) despite slower absorption demonstrates that route selection can critically impact drug concentration at the target site [19]. Furthermore, the potential for altered elimination kinetics between routes, as evidenced by chloramphenicol's longer half-life after oral administration, underscores the necessity for therapeutic drug monitoring when switching administration routes during treatment [19].
From a clinical perspective, the first-pass effect significantly influences oral bioavailability and thus dosing requirements. As observed with topotecan, oral administration results in substantially lower systemic exposure of the active lactone form (bioavailability ~20%) compared to intravenous administration [75]. This metabolic hurdle often necessitates higher oral doses to achieve therapeutic effects equivalent to IV administration, though this must be balanced against potential increases in metabolite-related toxicity [22]. Emerging approaches include the investigation of alternative administration routes such as subcutaneous delivery, which for topotecan demonstrated a favorable bioavailability profile (88.05% for lactone form) with sustained release characteristics [75].
The comparative analysis of intravenous versus oral administration reveals a complex interplay of pharmacokinetic parameters that directly inform regulatory review and clinical practice. Intravenous administration offers complete bioavailability and rapid onset but requires healthcare professional administration and carries higher risk of immediate adverse effects. Oral administration provides patient convenience and cost-effectiveness for chronic therapy but exhibits variable absorption and significant first-pass metabolism for many compounds. The experimental data and methodologies presented in this guide provide researchers and drug development professionals with evidence-based frameworks for route selection optimization. As pharmaceutical sciences advance, the integration of sophisticated modeling approaches like PBPK and exploration of alternative administration routes will further enhance our ability to individualize therapy based on comprehensive pharmacokinetic understanding.
Conclusion
The comparative pharmacokinetics of oral and intravenous administration present a complex landscape with direct implications for drug efficacy, safety, and development strategy. The foundational principles highlight the inherent trade-offs: IV administration offers complete bioavailability and precision, while oral administration provides convenience at the cost of variable absorption influenced by multifaceted biological and physicochemical barriers. Methodological advances, particularly PBPK modeling and microtracer studies, provide powerful tools for de-risking development and obtaining critical human PK data early. Troubleshooting efforts continue to evolve, with novel formulation technologies and personalized medicine approaches offering promising pathways to overcome the historical limitations of oral delivery. Ultimately, the choice of administration route must be a strategic decision informed by robust comparative validation, balancing pharmacological objectives with patient-centric outcomes. Future directions will likely see increased integration of in silico predictions with patient-specific factors, further blurring the lines between traditional routes through innovative delivery systems that optimize the pharmacokinetic-profile for improved therapeutic success.
References
- 1. BI 425809 in Healthy Males - PMC - PubMed Central [https://pmc.ncbi.nlm.nih.gov/articles/PMC8901509/]
- 2. Assessment of the absorption, metabolism and absolute ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2311408/]
- 3. Intravenous vs. Oral Dose Comparison of Ibuprofen and ... [https://www.mdpi.com/1424-8247/18/3/331]
- 4. Pharmacokinetics of FK 506 Following Oral Administration [https://pmc.ncbi.nlm.nih.gov/articles/PMC3032435/]
- 5. Comparative Pharmacokinetics, Bioequivalence and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6996485/]
- 6. Comparative pharmacokinetics of osmotic-controlled and ... [https://www.nature.com/articles/s41598-020-58801-1]
- 7. Advances in Oral Drug Delivery [https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2021.618411/full]
- 8. Drug Absorption - StatPearls - NCBI Bookshelf - NIH [https://www.ncbi.nlm.nih.gov/books/NBK557405/]
- 9. 1.3 Pharmacokinetics – Absorption [https://opentextbc.ca/nursingpharmacology/chapter/1-3-absorption/]
- 10. Why Your Body Processes Different Drugs at Varying Speeds [https://pointofcarenano.com/the-science-of-drug-absorption-why-your-body-processes-different-drugs-at-varying-speeds/]
- 11. Drug Absorption - Clinical Pharmacology [https://www.merckmanuals.com/professional/clinical-pharmacology/pharmacokinetics/drug-absorption]
- 12. Carrier-mediated transport and efflux mechanisms in Caco- ... [https://www.sciencedirect.com/science/article/pii/S0169409X96004140]
- 13. Advances and challenges in research methods on oral ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12449861/]
- 14. Predicting Food Effect On Oral Drug Absorption For ... [https://link.springer.com/article/10.1007/s11095-025-03947-8]
- 15. First-Pass Effect - StatPearls - NCBI Bookshelf - NIH [https://www.ncbi.nlm.nih.gov/books/NBK551679/]
- 16. First pass effect [https://en.wikipedia.org/wiki/First_pass_effect]
- 17. Drug Metabolism - StatPearls - NCBI Bookshelf [https://www.ncbi.nlm.nih.gov/books/NBK442023/]
- 18. A comprehensive review of the strategies to improve oral ... [https://www.sciencedirect.com/science/article/pii/S1773224720314672]
- 19. Pharmacokinetic comparison of intravenous and oral ... [https://pubmed.ncbi.nlm.nih.gov/6973130/]
- 20. Pharmacokinetics analysis results are similar for oral ... [https://www.nature.com/articles/s41409-019-0521-5]
- 21. Bioavailability Enhancement Techniques for Poorly Aqueous ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9495787/]
- 22. What is the difference between IV and PO pharmacokinetic ... [https://synapse.patsnap.com/article/what-is-the-difference-between-iv-and-po-pharmacokinetic-studies]
- 23. Applying Biopharmaceutical Classification System (BCS ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4476996/]
- 24. Link between drug absorption solubility and permeability ... [https://www.sciencedirect.com/science/article/pii/S0022354915507041]
- 25. Precipitation as key parameter for early oral drug ... [https://pubmed.ncbi.nlm.nih.gov/40691878/]
- 26. Precipitation as key parameter for early oral drug ... [https://www.sciencedirect.com/science/article/pii/S092809872500209X]
- 27. Food Effects on Oral Drug Absorption - PubMed Central - NIH [https://pmc.ncbi.nlm.nih.gov/articles/PMC7408216/]
- 28. Gastric transit and small intestinal transit time and motility ... [https://bmcgastroenterol.biomedcentral.com/articles/10.1186/1471-230X-11-145]
- 29. How to Assess Regional and Whole Gut Transit Time With ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4015195/]
- 30. A Systematic Review of Gastric Acid-Reducing Agent ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7109143/]
- 31. Intravenous vs. Oral Dose Comparison of Ibuprofen and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11944613/]
- 32. Impact of gastrointestinal tract variability on oral drug ... [https://www.sciencedirect.com/science/article/pii/S0928098721001147]
- 33. GLP-1RA-induced delays in gastrointestinal motility - PubMed [https://pubmed.ncbi.nlm.nih.gov/39989027/]
- 34. Predicting Oral Drug Absorption: Mini Review on ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5750647/]
- 35. Physiologically Based Pharmacokinetic (PBPK) Modeling ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4613950/]
- 36. Quality Assurance of PBPK Modeling Platforms and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9314283/]
- 37. Optimization of Bottomâ€Up PBPK Model Development in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12631063/]
- 38. Advances in Physiologically Based Pharmacokinetic ... [https://link.springer.com/article/10.1007/s11095-025-03849-9]
- 39. PBPK MODELING - Critical Parameters for Simulating Oral ... [https://drug-dev.com/pbpk-modeling-critical-parameters-for-simulating-oral-absorption-using-pbpk-models/]
- 40. Absolute Bioavailability And IV Pharmacokinetics [https://www.pharmaron.com/services/clinical-development/integrated-radiolabelled-sciences/accelerator-mass-spectrometry-ams/absolute-bioavailability-and-iv-pharmacokinetics/]
- 41. Microtracerâ€Based Assessment of the Mass Balance ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12402879/]
- 42. The application of Phase 0 and microtracer approaches in ... [https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2024.1369079/full]
- 43. Zabedosertib, a novel interleukin-1 receptor-associated ... [https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2025.1521505/full]
- 44. The Absolute Bioavailability and Absorption, Metabolism ... [https://www.sciencedirect.com/science/article/abs/pii/S0090955624010614]
- 45. A Physiologically Based Pharmacokinetic ( ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12358303/]
- 46. Intravenous vs. Oral Dose Comparison of Ibuprofen and ... [https://pubmed.ncbi.nlm.nih.gov/40143110/]
- 47. The PhASAge Study [https://pubmed.ncbi.nlm.nih.gov/40567999/]
- 48. Oral Cephalexin Population Pharmacokinetics and Target ... [https://pubmed.ncbi.nlm.nih.gov/40995926/]
- 49. Oral or intravenous antibiotics? - Australian Prescriber [https://australianprescriber.tg.org.au/articles/oral-or-intravenous-antibiotics.html]
- 50. Oral vs IV antibiotics - Therapeutics Letter - NCBI Bookshelf [https://www.ncbi.nlm.nih.gov/books/NBK615102/]
- 51. The Ultimate Guide to Regulatory Submissions - [https://pharmuni.com/2025/06/02/the-ultimate-guide-to-regulatory-submissions/]
- 52. Faster regulatory submissions for pharma with AI [https://www.mckinsey.com/industries/life-sciences/our-insights/rewiring-pharmas-regulatory-submissions-with-ai-and-zero-based-design]
- 53. Use of Physiologically Based Pharmacokinetic (PBPK ... - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC7520419/]
- 54. Food Effect Projections via Physiologically Based ... [https://www.sciencedirect.com/science/article/abs/pii/S0022354918303320]
- 55. Application of Physiologically-Based Pharmacokinetic (PBPK ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12334468/]
- 56. Applied Concepts in PBPK Modeling: How to Build a PBPK ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5080648/]
- 57. Difference between PBPK & Population PK Modeling [https://www.allucent.com/resources/blog/what-difference-between-pbpk-and-poppk-modeling]
- 58. Evaluation of the Effect of Food on the Pharmacokinetics ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9167418/]
- 59. Prediction of first-in-human dose for new composition bee ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11976151/]
- 60. More Accurate Prediction of Human Pharmacokinetic ... [https://dmpkservice.wuxiapptec.com/articles/438-more-accurate-prediction-of-human-pharmacokinetic-parameters-for-small-molecule-compounds-methods-models-and-applications/]
- 61. Pharmaceutical approaches for enhancing solubility and ... [https://www.sciencedirect.com/science/article/abs/pii/S0939641124003394]
- 62. Evaluating the Role of Solubility in Oral Absorption ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7158207/]
- 63. Drug Nanocarriers for Pharmaceutical Applications - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC12388938/]
- 64. Switch over from intravenous to oral therapy [https://pmc.ncbi.nlm.nih.gov/articles/PMC4008927/]
- 65. Nanocarrier Drug Delivery Systems: Characterization ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9026217/]
- 66. Drug solubility in lipid nanocarriers: Influence of lipid matrix ... [https://www.sciencedirect.com/science/article/abs/pii/S0378517317306245]
- 67. Pharmaceutical amorphous solid dispersion: A review of ... [https://www.sciencedirect.com/science/article/pii/S2211383521001805]
- 68. A Review on Solid Dispersion and Carriers Used Therein ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7335980/]
- 69. Grand challenges in oral drug delivery [https://www.frontiersin.org/journals/drug-delivery/articles/10.3389/fddev.2025.1571982/full]
- 70. Medication Routes of Administration - StatPearls - NCBI - NIH [https://www.ncbi.nlm.nih.gov/books/NBK568677/]
- 71. Solid Self-Microemulsifying Drug Delivery System for ... [https://pubmed.ncbi.nlm.nih.gov/39886543/]
- 72. Intravenous Drug Administration - an overview [https://www.sciencedirect.com/topics/medicine-and-dentistry/intravenous-drug-administration]
- 73. Intravenous Administration [https://nurseslabs.com/intravenous-administration/]
- 74. Glyceride-Mimetic Prodrugs Incorporating Self-Immolative Spacers... [https://pubmed.ncbi.nlm.nih.gov/27482655/]
- 75. Pharmacokinetic Comparison of Three Different ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7559546/]
- 76. The Role of Oral Controlled in Drug Delivery... Release Matrix Tablets [https://pmc.ncbi.nlm.nih.gov/articles/PMC3648939/]
- 77. Oral Dosage Forms: Some Recent Advances in... Controlled Release [https://scialert.net/fulltext/?doi=jms.2001.350.354]
- 78. Comparison of Release-Controlling Efficiency of Polymeric ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2902312/]
- 79. Targeting Receptors, Transporters and Site of Absorption to ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3155234/]
- 80. Orally Administered Drugs and Their Complicated ... [https://www.mdpi.com/2076-2607/12/2/242]
- 81. Advances in Pharmacokinetic Mechanisms of Transporter- ... [https://www.mdpi.com/1424-8247/15/9/1126]
- 82. Oral or intravenous antibiotics? - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC7186270/]
- 83. Impact of gastrointestinal tract variability on oral drug ... [https://strathprints.strath.ac.uk/76207/]
- 84. Physiological Dynamics in the Upper Gastrointestinal Tract ... [https://pubmed.ncbi.nlm.nih.gov/37783928/]
- 85. Recent advancement in polymeric nanoparticles for oral ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12579739/]
- 86. Pharmacokinetic considerations and challenges in oral ... [https://pharmaceutical-journal.com/article/research/pharmacokinetic-considerations-and-challenges-in-oral-anticancer-drug-therapy]
- 87. Hyundai ADM Bio Showcases Innovative Oral ... [https://biopharmaapac.com/news/18/5855/hyundai-adm-bio-showcases-innovative-oral-chemotherapy-otx-m-at-biotech-showcase-2025.html]
- 88. Analyzing Trends in Oral Anticancer Agents in an ... [https://jhoponline.com/issue-archive/2015-issues/june-vol-5-no-2/analyzing-trends-in-oral-anticancer-agents-in-an-academic-medical-facility]
- 89. Oral Bioavailability Enhancement of Anti-Cancer Drugs ... [https://pubmed.ncbi.nlm.nih.gov/40143044/]
- 90. Smart nanoparticle delivery systems for resveratrol [https://www.sciencedirect.com/science/article/abs/pii/S0026265X25026554]
- 91. ESMO 2025: Oral drug demonstrates promising anti-tumor ... [https://www.mdanderson.org/newsroom/research-newsroom/esmo-2025--oral-drug-demonstrates-promising-anti-tumor-activity-.h00-159780390.html]
- 92. What Are the Latest Oral Chemotherapy Options in 2025? [https://continentalhospitals.com/blog/what-are-the-latest-oral-chemotherapy-options-in-2025/]
- 93. Modeling of pharmacokinetic/pharmacodynamic parameters ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12276709/]
- 94. Comparative Pharmacokinetics and Safety of a Micellar ... [https://www.mdpi.com/2076-3921/14/11/1313]
- 95. Comparison of Intravenous and Oral Meloxicam ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12390456/]
- 96. Pharmacokinetics of difloxacin after single intravenous ... [https://www.sciencedirect.com/science/article/pii/S0032579125000379]
- 97. Pharmacokinetics - StatPearls - NCBI Bookshelf - NIH [https://www.ncbi.nlm.nih.gov/books/NBK557744/]
- 98. The use of pharmacokinetic and pharmacodynamic data in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC1884636/]
- 99. Pharmacokinetics and Pharmacodynamics in Drug ... [https://amsbiopharma.com/pk-pd-studies-drug-development-adme/]
- 100. Comparison of the Safety and Pharmacokinetics of ST-246 ... [https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0023237]
- 101. Comparison of pharmacokinetics software for therapeutic drug ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11042351/]
- 102. Drug Interaction PBPK Modeling: Review of the Literature ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12267664/]
- 103. Impact of route-dependent phase-II gut metabolism and ... [https://www.sciencedirect.com/science/article/pii/S0753332222005303]
- 104. A review of animal models utilized in preclinical studies of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11034049/]
- 105. Human disease models in drug development [https://www.nature.com/articles/s44222-023-00063-3]
- 106. Preclinical Assessment for Translation to Humans (PATH) [https://pmc.ncbi.nlm.nih.gov/articles/PMC11471378/]
- 107. Preclinical Safety vs Efficacy Research [https://www.itrlab.com/preclinical-safety-vs-efficacy-research/]
- 108. Statistical analysis and reporting of clinical pharmacokinetic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9372427/]
- 109. Oral versus intravenous antibiotics for bone and joint ... [https://www.sciencedirect.com/science/article/abs/pii/S8756328225001061]
- 110. Systematic review: the bioavailability of orally administered ... [https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-021-05919-w]
- 111. Implementation of oral versus intravenous antibiotics in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12424555/]
- 112. A Look at the Evidence: Should Orthopaedic Surgeons ... [https://www.aaos.org/aaosnow/2025/june/clinical/clinical06/]
- 113. Oral versus intravenous antibiotics for bone and joint ... [https://pubmed.ncbi.nlm.nih.gov/40280255/]
- 114. Development of a multi-route physiologically based ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9264615/]
- 115. Visualization and Communication of Pharmacometric Models ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4055786/]